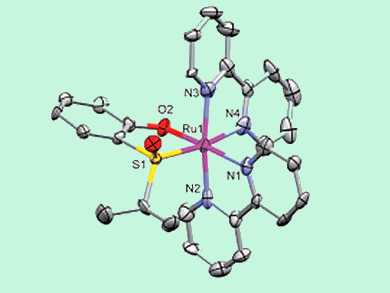
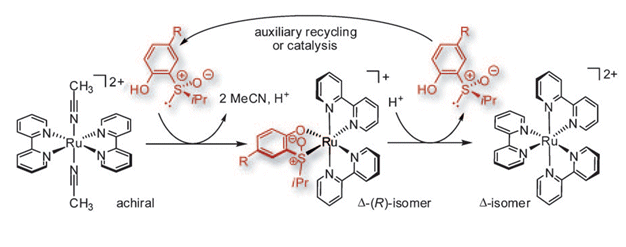
The research group of Meggers, who are based in Marburg, Germany, has reported the first example of enantioselective synthesis of “chiral-at-metal” ruthenium complexes mediated by a chiral sulfoxide auxiliary.
This proof-of-principle study shows that chiral (S)-(isopropylsulfinyl)phenol is capable of inducing (and even catalyzing) conversion of achiral trans-[Ru(bpy)2(MeCN)2]2+ into the chiral cis-Δ-[Ru(bpy)2{(S)-(isopropylsulfinyl)phenolato}]+ species (see scheme). Ingenious reaction conditions were employed to keep the starting material out of solution and help secure a good level of enantioselectivity.
The results of this study remove the necessity to resolve racemates, thus making the preparation of these six-coordinate complexes more atom-efficient.
- Isomerization-Induced Asymmetric Coordination Chemistry: From Auxiliary Control to Asymmetric Catalysis
L. Gong, Z. Lin, K. Harms, E. Meggers,
Angew. Chem. Int. Ed. 2010, 49.
DOI: 10.1002/anie.201003139 - L. Gong, Z. Lin, K. Harms, E. Meggers,
Angew. Chem. 2010, 122.
DOI: 10.1002/ange.201003139