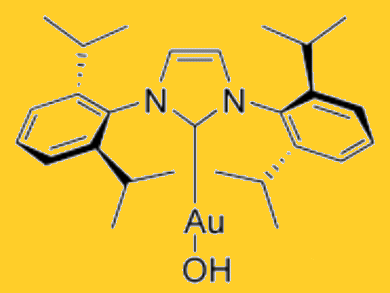
To improve the efficiency of gold catalysts, weakly coordinating anions are used to increase the Lewis acidity of the cationic gold center. Silver salts are generally used to perform the anion exchange; however these are often moisture or light sensitive.
Steven Nolan and co-workers, University of St Andrews, Scotland, use the air- and moisture-stable precatalyst [Au(OH)(IPr)] (IPr=N,N’-bis(2,6-diisopropylphenyl)-imidazol-2-ylidene) in a silver-free route to the [Au(IPr)]+ ion.
Addition of one equivalent of Brønsted acid, tetrafluoroboric acid–etherate (HBF4•OEt2), generated the cationic [Au(IPr)]+ species as evidenced by 1H NMR. The dinuclear gold hydroxide intermediate [{Au(IPr)}2(μ-OH)] was observed on addition of HBF4 and this was suggested as a reservoir for the active catalyst, [Au(IPr)][BF4].
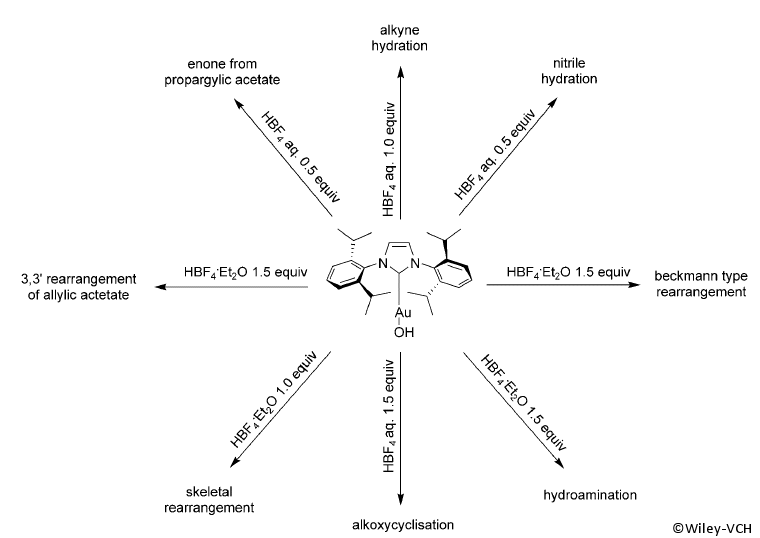
The use of [Au(IPr)][BF4] in a range of catalytic reactions was also demonstrated (see figure).
- Development of Versatile and Silver-Free Protocols for Gold(I) Catalysis
S. Gaillard, J. Bosson, R. S. Ramón, P. Nun, A. M. Z. Slawin, S. P. Nolan,
Chem. Eur. J. 2010, 16.
DOI: 10.1002/chem.201001688