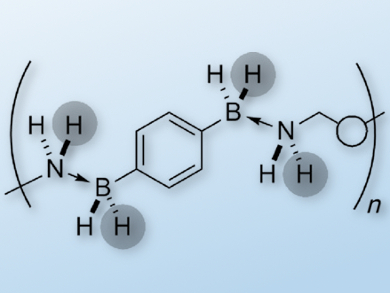
The use of ammonia borane (NH3·BH3) as a dihydrogen storage material is theoretically promising as it has a high H2 storage capacity. However, its practical application has several pitfalls such as poor processability. Polymeric boron nitrides are therefore interesting alternative materials for H2 storage.
Jean Raynaud, Emmanuel Lacôte, and colleagues, Université de Lyon, France, set out to synthesize amine- and borane-containing polymers that can release H2 on demand. They came up with a novel method of assembling the polymers: instead of covalent bonds, they use multiple Lewis pairings in the polymer backbone. The polymeric amine boranes show substantially different behavior from their molecular analogues.
The polymers undergo endothermic H2 release at a temperature that is lower than that for discrete molecules, and they are also more effective than their molecular counterparts in transfer hydrogenation reactions of imines, ketones, and aldehydes. The researchers also carried out computational studies in order to understand the special behavior of the polymers. These polymers are a promising starting point in the development of recyclable hydrogen storage materials.
- Polyboramines for Hydrogen Release: Polymers Containing Lewis Pairs in their Backbone,
Audrey Ledoux, Paolo Larini, Christophe Boisson, Vincent Monteil, Jean Raynaud, Emmanuel Lacôte,
Angew. Chem. Int. Ed. 2015, 54, 15744–15749.
DOI: 10.1002/anie.201508395