It has previously been reported that under a pressure of greater than 110 GPa, H2S is converted into a superconductor at a remarkably high temperature of approximately 200 K.
The groups of Arndt Simon and Jürgen Kohler, both at the Max-Planck-Institut für Festkörperforschung, Stuttgart, Germany, and Myung-Hwan Whangbo, North Carolina State University, USA, now propose that this superconducting phase is not body-centered cubic H3S, as previously surmised, but that it is formed by the decomposition of 2 H2S into H3S+ and SH–.
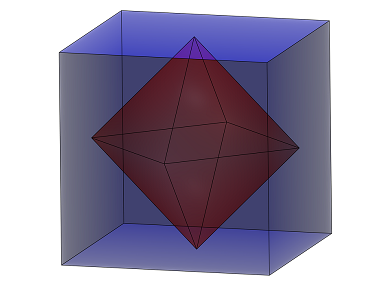
This process, which was suggested in analogy to the dissociation of water into H3O+ and OH–, leads to the formation of the perovskite (SH–)(H3S+), which consists of corner-sharing SH6 octahedra with an SH– ion at the center of each S8 cube. Calculations suggest that the hydrogen atoms of these SH– ions are fluxional even at the critical temperature.
- Structure and Composition of the 200 K-Superconducting Phase of H2S at Ultrahigh Pressure: The Perovskite (SH–)(H3S+),
E. E. Gordon, K. Xu, H. Xiang, A. Bussmann-Holder, R. K. Kremer, A. Simon, J. Köhler, M.-H. Whangbo,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201511347
Previous Report
- Superconductivity Record
ChemistryViews.org, August 2015.
New high temperature record for conducting electricity without resistance - Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system,
A. P. Drozdov, M. I. Eremets, I. A. Troyan, V. Ksenofontov, S. I. Shylin,
Nature 2015, 525, 73–76.
DOI: 10.1038/nature14964