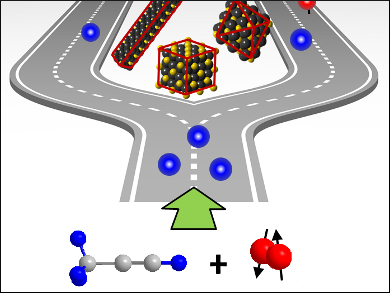
Parahydrogen induced polarization (PHIP) is a nuclear magnetic resonance (NMR) signal enhancement technique with potential applications in clinical magnetic resonance imaging (MRI). PHIP-NMR can provide unique information about hydrogenation mechanisms that is not available from any other analytical technique. This is due to the fact that PHIP-NMR signals are observed only when the two hydrogen atoms that are transferred to the unsaturated substrate originate from the same parahydrogen (H2) molecule, i.e., when the hydrogenation occurs by pairwise addition.
Clifford R. Bowers and Helena E. Hagelin-Weaver, University of Florida, Gainesville, USA, and colleagues used PHIP-NMR to investigate the selective hydrogenation of propyne to propene over shaped CeO2 nanocrystals, an industrially important reaction to remove alkynes from alkene streams. The dominant reaction path for semihydrogenation of propyne over ceria {1 1 1} proceeds via a low-energy six-membered-ring transition state. CeO2 nanocrystals show a cubic fluorite crystal structure. Ceria nanorods are enclosed predominantly by {1 1 0} and {1 0 0} facets, nano-octahedra and nanocubes expose (111) and (100) facets, respectively. A facet-structure dependence of the total activity and pairwise selectivity were observed.
Whereas the CeO2 octahedra are most active in the hydrogenation of propyne, pairwise selectivity is significantly higher over the rods. In contrast, the pairwise selectivity of propene hydrogenation over CeO2 was previously shown to be facet-independent. These observations, which are consistent with recent DFT calculations, show the potential of PHIP-NMR for the study of heterogeneous hydrogenation reactions.
- Semihydrogenation of Propyne over Cerium Oxide Nanorods, Nanocubes, and Nano-Octahedra: Facet-Dependent Parahydrogen-Induced Polarization,
Evan W. Zhao, Yan Xin, Helena E. Hagelin-Weaver, Clifford R. Bowers,
ChemCatChem 2016.
DOI: 10.1002/cctc.201600270