To understand the role of group 4 metallocenes (Ti, Zr, and Hf) as important intermediates in catalytic reactions, they must first be studied on a stoichiometric level. In this context, metallocenes have been used to activate small unsaturated molecules and couple them together. Isonitrile is one such molecule of interest that allows for a wide reactivity profile of group 4 metallocenes to be established, and thus improve the comprehension of metallocene chemistry.
Uwe Rosenthal, Leibniz Institute for Catalysis e. V. at the University of Rostock, Germany, and colleagues have performed an extensive study of group 4 metallocene–alkyne reactivity with 2-xylyl isonitrile. The key transformations reported were stoichiometric C-C and C-N couplings within the group 4 metal coordination sphere (i.e., at the metal) to form heterometallacycles incorporating nitrogen.
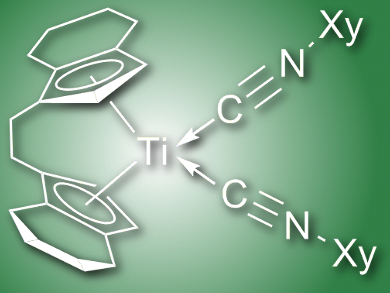
The nature of the metal and the substituted cyclopentadienyl ligand, the corresponding stoichiometry, and the reaction temperature and time all proved to have an influence on the resulting metallocene product. Upon reaction of different metallocenes with one or more 2-xylyl isonitriles, the resulting metallacycle products included end-on coordination of 2-xylyl isocyanide (pictured), enimine complexes, aza-metallocycles, and fused heterocyclic systems. Zirconium heterometallacycles were found to undergo interconversion between mono- and tricyclic products, as observed by 1H NMR.
- Multiple and Highly Selective Alkyne-Isonitrile C−C and C−N Couplings at Group 4 Metallocenes,
Kai Altenburger, Perdita Arndt, Lisanne Becker, Fabian Reiß, Vladimir V. Burlakov, Anke Spannenberg, Wolfgang Baumann, Uwe Rosenthal,
Chem. Eur. J. 2016, 22, 9169–9180.
DOI: 10.1002/chem.201601465