Finding the Texture of Crystals
Whether building bone, shells, or corals, living creatures are true masters of crystallization. In the laboratory, this amazing precision cannot be duplicated yet. The processes and many of the precise structures of biominerals remain largely unexplored. In the journal Angewandte Chemie, an international team has now introduced a three-dimensional X-ray diffraction technique for determining crystallographic texture – the preferred orientation of the little crystals in a solid – with previously unattainable spatial resolution.
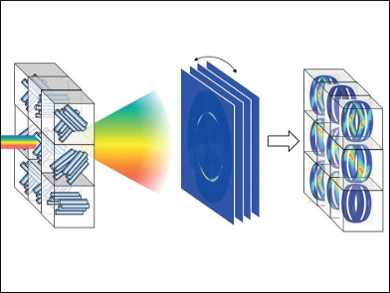
Whether mineral, biomineral, or inorganic material, the arrangement of the individual crystallites has a large influence on its properties. The texture of a sample can be determined by means of X-ray diffraction. X-rays are bounced off the electron shells of the atoms. The waves emanating from the individual atoms interfere, resulting in a diffraction pattern that depends on the distances between the atoms and their relative locations. These patterns are recorded by a detector and make it possible to draw conclusions about the crystallite’s structure. However, this conventional method of analysis only provides a projection, that is, two-dimensional data. It is possible to get three-dimensional information by rotating the sample within the beam, but this changes the irradiated sample volume, which causes the signals to smear. It is difficult to examine complex structures by this method.
Three-Dimensional Analysis
A team of researchers from the University of Natural Resources and Life Sciences (BOKU) in Vienna, Austria; Ghent University, Belgium; the German Electron Synchrotron, Hamburg; the European Synchrotron Radiation Facility (ESRF) in Grenoble, France; the University of Liverpool, UK; and the Netherlands Organization for Scientific Research (NWO) in Grenoble; has now overcome this problem.
The key to the team’s method is to irradiate the sample with “white” X-rays from a synchrotron. This means that the X-rays cover a spectrum of energies, rather than a single energy level. This method provides additional information because each energy is diffracted differently. By using a special camera, the diffraction pattern can be separated according to energy level, which makes it possible to resolve the corresponding spectrum in each individual pixel. Computations make it possible to translate the X-ray photon energy into the missing third dimension.
Examining Complex Structures
Led by Helga Lichtenegger, the researchers demonstrated the power of their technique on carbon fibers, which have a well-known structure. In addition, they examined the shells of the American lobster. These were thought to consist of layers of helically arranged chitin fibers mineralized with amorphous calcium carbonate. Calcite crystals were thought to be arranged perpendicular to the chitin layer. These assumptions have now been confirmed and refined.
“Our new method delivers direct 3D information with a single measurement – without previous knowledge of the sample,” says Lichtenegger. “It allows for texture analysis of large samples with complex substructures and may make it possible to follow textural changes in situ, e.g., during crystallization.”
- Photon Energy Becomes the Third Dimension in Crystallographic Texture Analysis,
Tilman A. Grünewald, Harald Rennhofer, Pieter Tack, Jan Garrevoet, Didier Wermeille, Paul Thompson, Wim Bras, Laszlo Vincze, Helga C. Lichtenegger,
Angew. Chem. Int. Ed. 2016.
DOI: 10.1002/anie.201603784