Lithium-ion batteries are commonly used in portable electronic devices. Their anodes store lithium ions, and the capacity and stability of the battery depend on the materials used. Carbon materials such as carbon nanotubes or graphene are examples of such materials. However, their agglomeration can reduce the efficiency of the anode. 3D networks made from carbon nanostructures, in contrast, prevent agglomeration and provide channels for ion migration.
Rodney S. Ruoff, National Institute of Science and Technology, Ulsan, Republic of Korea, Yanwu Zu, University of Science and Technology of China, Hefei, and colleagues have converted C60 into nitrogen-doped 3D porous carbon for applications as an anode material in Li-ion batteries. The team treated C60 with KOH in an ammonia atmosphere at 500–700 °C to prepare the N-doped carbon.
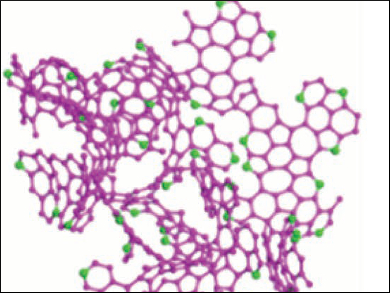
The resulting material is a 3D network of mostly sp2-hybridized carbon atoms with many defects and a large amount of both pyrrolic and pyridinic nitrogen atoms (pictured). The nitrogen doping causes a high volume of both meso- and macropores in the network. The porous carbon performs very well as an anode material with a reversible capacity of up to 1900 mAh/g at 100 mA/g, a good rate performance, and excellent cycling stability. According to the researchers, simulations show that both curved fullerene fragments and nitrogen doping contribute to the high Li-ion storage capacity.
- Incorporating Pyrrolic and Pyridinic Nitrogen into a Porous Carbon made from C60 Molecules to Obtain Superior Energy Storage,
Ziqi Tan, Kun Ni, Guanxiong Chen, Wencong Zeng, Zhuchen Tao, Mujtaba Ikram, Qiubo Zhang, Huijuan Wang, Litao Sun, Xianjun Zhu, Xiaojun Wu, Hengxing Ji, Rodney S. Ruoff, Yanwu Zhu,
Adv. Mater. 2016.
DOI: 10.1002/adma.201603414