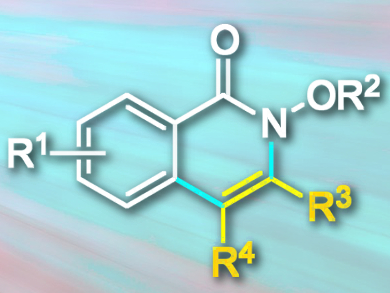
N-heterocycles are important building blocks in medicinal chemistry. Isoquinolone (derivative pictured) is an N-heterocyclic scaffold present in many alkaloids and biologically active molecules. The metal-catalyzed cyclization of aromatic amides and alkynes is one protocol for the synthesis of isoquinolone derivatives.
Govindasamy Sekar, Indian Institute of Technology, Madras, India, and colleagues have developed a method to synthesize isoquinolones from N-alkoxy benzamides and alkynes which uses binaphthyl-stabilized palladium nanoparticles (Pd-BNP) as a catalyst. Using a nanocatalyst makes the recovery and reuse of the catalyst easier. The team combined a range of N-alkoxy benzamides with a variety of alkynes in the presence of Pd-BNP, using KI as an additive, air as an oxidant, and dimethylformamide (DMF) as a solvent.
The developed reaction protocol gave a variety of isoquinolones in good to excellent yields. The approach could also be used to synthesize sulfur analogues of isoquinolones from N-methoxybenzothiamide in moderate yields. The Pd-BNP catalyst could easily be recovered by centrifugation and reused up to four times without agglomeration.
- Palladium-Nanoparticles-Catalyzed Oxidative Annulation of Benzamides with Alkynes for the Synthesis of Isoquinolones,
Nidhi Sharma, Rajib Saha, Naziya Parveen, Govindasamy Sekar,
Adv. Synth. Catal. 2017.
DOI: 10.1002/adsc.201601137