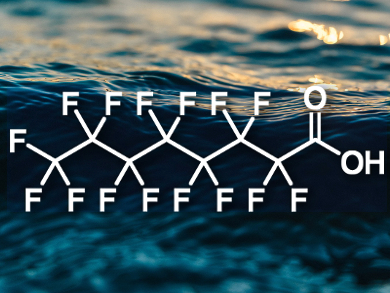
Perfluorinated compounds are used, e.g., in the production of Teflon and fire suppressants. They can accumulate in the environment and have negative effects on human health. Perfluorooctanoic acid (PFOA, pictured), in particular, can contaminate the groundwater near industrial sites. While activated carbon can remove this compound, it is easily fouled and rendered inactive by other compounds such as humic acids, which are produced by the biodegradation of organic matter and are ubiquitous in water.
Damian E. Helbling, Cornell University, Ithaca, NY, USA, William R. Dichtel, Cornell University and Northwestern University, Evanston, IL, USA, and colleagues have developed a β-cyclodextrin-based polymer network which can remove PFOA and is not affected by humic acids. The team crosslinked a β-cyclodextrin polymer with decafluorobiphenyl linkers using a nucleophilic aromatic substitution reaction.
The resulting polymer removed PFAO from contaminated water as fast as activated carbon and reduced the concentration below advised levels. The material’s affinity towards PFOA was over ten times higher than that of activated carbon, and it can be regenerated and reused by washing it with methanol. Humic acids did not affect the adsorption. According to the researchers, the good PFAO removal performance can be attributed to the polymer’s cyclodextrin binding sites.
- β-Cyclodextrin Polymer Network Sequesters Perfluorooctanoic Acid at Environmentally Relevant Concentrations,
Leilei Xiao, Yuhan Ling, Alaaeddin Alsbaiee, Chenjun Li, Damian E. Helbling, William R. Dichtel,
J. Am. Chem. Soc. 2017.
DOI: 10.1021/jacs.7b02381