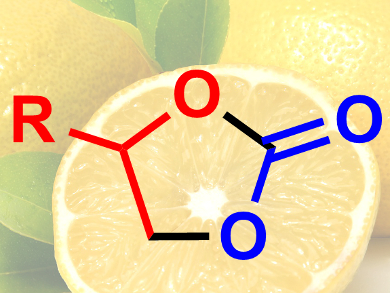
Cyclic organic carbonates are used as intermediates in the chemical industry. They can be synthesized by reacting epoxides with CO2. A low-cost, metal-free, sustainable catalyst for this reaction would be useful. While there are some organic catalysts that can promote this cyclization, few of them function efficiently under ambient conditions.
Valerio D’Elia, Vidyasirimedhi Institute of Science and Technology (VISTEC), Payupnai, Wang Chan, Thailand, and colleagues have discovered that ascorbic acid (vitamin C) can be used as a cheap and nontoxic hydrogen bond donor which activates epoxides. The team used an ascorbic acid/tetrabutylammonium iodide (TBAI) mixture to catalyze the conversion of epoxides to cyclic carbonates (pictured) at room temperature and under low CO2 pressure.
The reaction proceeds in good yields, which can be further improved by raising the temperature to 40 °C. The researchers performed density functional theory (DFT) calculations to investigate the reaction mechanism and found that the epoxide forms hydrogen bonds with the ascorbic acid’s hydroxyl groups. This activates the epoxide, which is then opened by an iodide ion stemming from TBAI, followed by the insertion of CO2 and ring closure. According to the team, ascorbic acid is promising for applications as a sustainable organocatalyst.
- Ascorbic Acid as a Bifunctional Hydrogen Bond Donor for the Synthesis of Cyclic Carbonates from CO2 under Ambient Conditions,
Sunatda Arayachukiat, Chutima Kongtes, Alexander Barthel, Sai V. C. Vummaleti, Albert Poater, Sippakorn Wannakao, Luigi Cavallo, Valerio D’Elia,
ACS Sustainable Chem. Eng. 2017.
DOI: 10.1021/acssuschemeng.7b01650