Iron plays a crucial role in a variety of vital cell functions, for example, in cell respiration and oxygen transportation in the human body. On the other hand, an iron overload can cause irreversible damage to cells and the over-consumption of iron supplements is potentially deadly. This shows that the iron household must be carefully balanced and iron chelators as drugs, as for example deferoxamine (DFO), must be used very carefully.
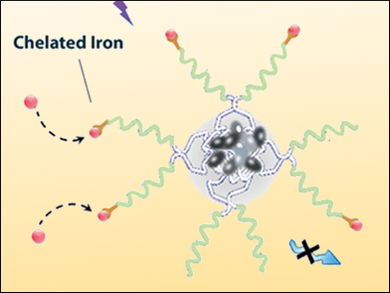
May P. Xiong, University of Georgia, Athens, USA, and colleagues have designed multifunctional self-assembling micelles composed of poloxamers, which are nonionic triblock copolymers built of a central hydrophobic chain of polyoxypropylene, flanked by two hydrophilic chains of polyoxyethylene. The team encapsulated tetraphenylethene (TPE) into the micelle core and conjugated the iron-chelating DFO to the surface of the micelles.
The resulting micelles retain the iron-chelation properties of DFO and exhibit only negligible cytotoxicity compared with free DFO. The crucial advantage of this multifunctional micelle is that, after the binding of DFO to Fe3+, the complex shows a near-ideal overlap between the absorption and fluorescence spectrum of TPE. Using the resulting fluorescence quenching, the chelation process of iron can be accurately monitored and the iron concentration in cells can be precisely adjusted.
- Multifunctional Polymeric Micelles for Combining Chelation and Detection of Iron in Living Cells,
Zhi Liu, Max Purro, Jing Qiao, May P. Xiong,
Adv. Healthcare Mater. 2017.
DOI: 10.1002/adhm.201700162