Halogenations are important reactions in organic chemistry. While there are many approaches to selective chlorination and bromination reactions, iodinations usually involve highly reactive reagents and are, thus, less selective.
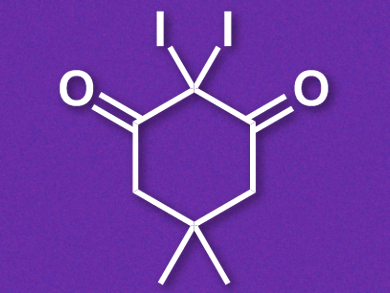
Belén Martén-Matute, Stockholm University, Sweden, and colleagues have developed a mild iodinating agent which can be used, e.g., in the electrophilic iodination of aromatic compounds and also in the selective synthesis of α-iodoketones from allylic alcohols. The team synthesized the reagent, 2,2-diiododimedone (pictured), by combining dimedone with iodine monochloride in 1,4-dioxane. This synthesis can easily be carried out on a gram scale and the product can be purified by a simple filtration.
The prepared reagent can then be used in iodination reactions together with iridium(III) complexes as a catalyst. The team achieved high yields and good selectivities under mild conditions (at room temperature in 16 hours). According to the researchers, the resulting α-iodocarbonyl compounds are useful intermediates for the selective synthesis of previously difficult-to-access organic structures.
- 2,2-Diiododimedone: a mild electrophilic iodinating agent for the selective synthesis of α-iodoketones from allylic alcohols,
Samuel Martinez-Erro, Antonio Bermejo Gómez, Ana Vázquez-Romero, Elis Erbing, Belén Martín-Matute,
Chem. Commun. 2017, 53, 9842–9845.
DOI: 10.1039/c7cc04823h

![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)

