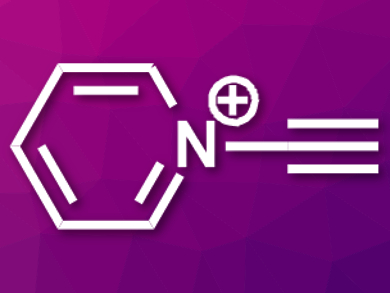
Cationic nitrogen-embedded polycyclic aromatic hydrocarbons (cNe-PAHs) are compounds with interesting physicochemical properties and a variety of potential applications. Methods for the synthesis of cNe-PAHs can suffer from problems such as low regioselectivity and a limited product range. Using N-alkynylpyridinium salts (cation pictured) as precursors could help researchers to circumvent these issues.
Naoyuki Toriumi, University of Tokyo, Japan, Masanobu Uchiyama, University of Tokyo and RIKEN, Saitama, Japan, and colleagues have achieved the first synthesis of N-alkynylpyridinium salts. The team used alkynyl-λ3-iodanes as alkynyl transfer reagents to prepare the desired N-alkynylpyridinium cations from different pyridine derivatives in good to excellent yields.
The N-alkynylpyridinium cations are highly electrophilic and undergo Michael additions with hydrogen halides and thiols to give adducts in high yields. The team was then able to convert these products to the respective cNe-PAHs using intramolecular cyclization reactions. According to the researchers, the method can be used to prepare a variety of cNe-PAHs with interesting optical and electrochemical properties.
- N-Alkynylpyridinium Salts: Highly Electrophilic Alkyne–Pyridine Conjugates as Precursors of Cationic Nitrogen-Embedded Polycyclic Aromatic Hydrocarbons,
Naoyuki Toriumi, Norihito Asano, Kazunori Miyamoto, Atsuya Muranaka, Masanobu Uchiyama,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b00356


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
