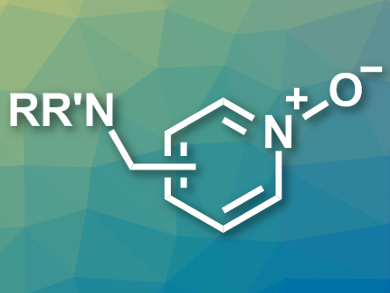
Enzymatic reactions can serve as inspiration for organic syntheses. In the oxidative metabolism, for example, there are selective, enzyme-catalyzed oxidations of nitrogen-containing heteroaromatic compounds that are difficult to achieve in a nonenzymatic way. This type of selective conversion is especially important for pharmaceutical chemistry, because bioactive compounds often contain amine groups which are oxidized more easily than the nitrogen in the heterocycles.
Michael K. Hilinski and colleagues, University of Virginia, Charlottesville, USA, have developed the first nonenzymatic heteroarene N-oxidation in the presence of amine groups. The team exploited the different basicities of the amine and the heteroarene and protected the more nucleophilic amine using a Brønsted acid (HBF4 or H2SO4). The heteroarene was then oxidized using H2O2 in the presence of an iminium salt as an organocatalyst. The protocol selectively gives the desired heteroaromatic N-oxides (example pictured) in good yields.
The researchers successfully used the developed method to mimic the oxidation of drug molecules by the enzyme aldehyde oxidase. According to the team, the method is suitable for late-stage functionalization and should complement existing oxidation approaches, as it reverses the usual N-oxidation selectivity.
- Selective Heteroaryl N-Oxidation of Amine-Containing Molecules,
Robert M. B. Dyer, Philip L. Hahn, Michael K. Hilinski,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b00558