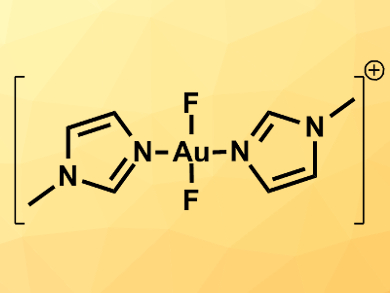
There a very few examples of well-defined gold(III) fluoride complexes. However, such compounds could be useful in catalysis.
Jason L. Dutton and colleagues, La Trobe University, Melbourne, Australia, have synthesized trans-difluoride complexes of gold; only the second example of this type of compound to be characterized. The team combined N-methylimidazole with chlorotetrahydrothiophene gold and KOTf (OTf = triflate) in dichloromethane. The mixture was stirred for 24 hours and the product was converted to the desired difluorogold complex using XeF2 in chloroform. Alternatively, KF could be used as a cheaper, less toxic fluoride source giving lower yields.
The complex was characterized using X-ray crystallography. Tthe team found that it features the shortest Au–F bond distance in a gold complex measured so far. The researchers were also able to synthesize similar complexes with pyridine ligands.
- Well defined difluorogold(III) complexes supported by N-ligands,
Jason Laurence Dutton, Mohammad Al Bayer, Robert Corbo,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc02535e