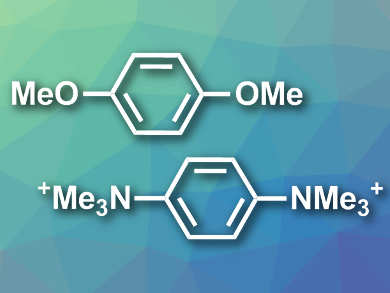
Polymers with many conjugated π-bonds, such as polyarenes, are useful, e.g., for applications in optoelectronic materials. They can be synthesized using cross-coupling reactions between dimetallic aromatic compounds (e.g., Grignard compounds) and dihaloarenes. Less reactive aromatic species with C–O or C–N bonds (examples pictured) are easy to access and, thus, could be useful replacements for dihaloarenes in this type of reaction.
Chao Wang, Masanobu Uchiyama, University of Tokyo and Advanced Elements Chemistry Laboratory, RIKEN, Saitama, both Japan, and colleagues have developed a Ni/Pd-catalyzed cross-coupling reaction that uses ethers or ammonium salts as reactants and proceeds via the cleavage of relatively inert C–O or C–N bonds. The team combined Grignard reagents or organolithium compounds with aryl methyl ethers or quaternary aryl ammonium salts in the presence of commercially available Ni or Pd catalysts such as NiCl2(PCy3)2, Ni(cod)2, or Pd(PPh3)2Cl2 (PCy3 = tricyclohexylphosphine, cod = 1,5-cyclooctadiene).
The developed reactions proceed under mild conditions and give the desired polymers with high molecular weights and in good to excellent yields. In addition, the approach works with monomers that are unsuitable for other polymerization methods.
- Cross-coupling polycondensation via C–O or C–N bond cleavage,
Ze-Kun Yang, Ning-Xin Xu, Ryo Takita, Atsuya Muranaka, Chao Wang, Masanobu Uchiyama,
Nat. Commun. 2018.
https://doi.org/10.1038/s41467-018-03928-z