Transition-metal-catalyzed cross-coupling reactions are very useful tools in organic synthesis. However, they often require expensive metals such as palladium. Nickel is a good alternative with a lower cost and good catalytic activity.
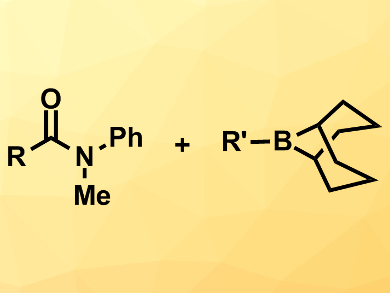
Magnus Rueping, RWTH Aachen University, Germany, and King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia, and colleagues have developed a nickel-catalyzed cross-coupling of amides with alkylboranes under mild conditions. The team combined a range of N-arylated amides with a variety of B-alkyl-9-BBNs (pictured right, 9-BBN = 9-borabicyclo[3.3.1]nonane) using Ni(cod)2 (cod = 1,5-cyclooctadiene) as a catalyst, an N-heterocyclic carbene (NHC) as a ligand, K2CO3 as a base, and LiCl as an additive. The reactions were performed in diisopropyl ether at 90 °C.
The desired products were obtained in good to high yields. The reaction tolerates a range of functional groups both at the amide and at the alkylborane, even carboxylic acid-derived groups such as esters.
- Cross-Coupling of Amides with Alkylboranes via Nickel-Catalyzed C–N Bond Cleavage,
Xiangqian Liu, Chien-Chi Hsiao, Lin Guo, Magnus Rueping,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b01021