Metal-organic frameworks (MOFs) are porous materials with a wide range of applications. They are held together by relatively weak interactions, which results in large numbers of degrees of freedom within the networks. This can cause a rare effect: negative thermal expansion (NTE), meaning the material contracts when it is heated. Usually, this contraction appears in only one or two dimensions. Three-dimensional NTE is rare and usually only found in high-symmetry cubic phases of MOFs.
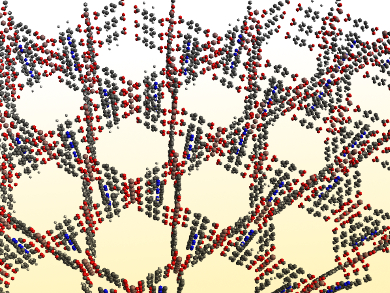
Xianran Xing and colleagues, University of Science and Technology Beijing, China, have found the first example of 3D anisotropic NTE in a low-symmetry MOF. The team studied the behavior of the MOF MIL-68(In), which has an orthorhombic structure (pictured). They activated the MOF at 220 °C overnight to remove guest molecules. Then the researchers used high-energy synchrotron X-ray diffraction (SXRD) and single crystal X-ray diffraction (SCXRD) to observe changes in the material’s structure upon heating.
MIL-68(In) shows an anisotropic NTE between 125 K and 600 K, with coefficients of thermal expansion along the three axes of αa = –5.6·10–6 K–1, αb = –2.7·10–6 K–1, and αc = –4.0·10–6 K–1, respectively. The X-ray diffraction results show that the contraction in the ab plane is due to a vibration of phenyl rings in the MOF’s organic ligands, while the contraction along the c axis can be attributed to a rotation of rigid indium octahedrons. According to the researchers, these insights could help with tailoring the thermal expansion of MOF materials and with designing new NTE materials.
- 3D negative thermal expansion in orthorhombic MIL-68(In),
Zhanning Liu, Qiang Li, He Zhu, Kun Lin, Jinxia Deng, Jun Chen, Xianran Xing,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc03219j