Graphene is a versatile 2D material used in various applications. Whilst it is useful in its two-dimensional form, researchers have started to create new 3D structures by linking graphene sheets together into different structures.
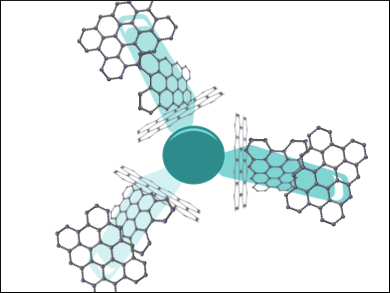
Michael L. Steigerwald, Thomas J. Sisto, Columbia University, New York, USA, Colin Nuckolls, Columbia University and Wuhan University of Science and Technology, China, Luis Echegoyen, University of Texas at El Paso, USA, and colleagues have created a new concept for designing 3D graphene nanostructures. The team used graphene nanoribbons as “spokes” and connected them covalently to a triptycene derivative that acts as a 3D molecular “hub”. The connections were formed using a Pd-catalyzed coupling reaction. The researchers created three different propeller-shaped 3D graphene nanostructures. The structures have molecular weights up to 6652 g/mol.
The properties of these nanostructures are different from those of the individual nanoribbons. They can facilitate the delocalization of electrons across the entire surface of the nanostructure, resulting in efficient charge transport properties. The researchers tested the graphene nanostructures as the electron extraction layer in a perovskite solar cell. The largest nanostructure was found to be the most efficient, yielding a power conversion efficiency (PCE) of 18.0 %.
- Three-Dimensional Graphene Nanostructures,
Samuel R. Peurifoy, Edison Castro, Fang Liu, X.-Y. Zhu, Fay Ng, Steffen Jockusch, Michael L. Steigerwald, Luis Echegoyen, Colin Nuckolls, Thomas J. Sisto,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b04119