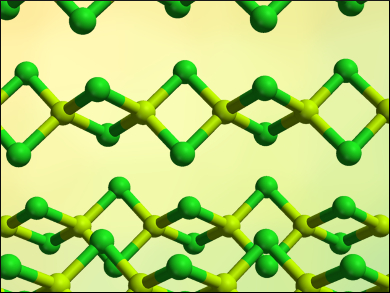
Beryllium and its compounds are toxic and carcinogenic, which is why research on these compounds has been limited. Beryllium chloride (structure pictured) is the only commercially available beryllium halide and the most common starting material for beryllium chemistry. However, many suppliers have stopped offering the compound. In addition, the bromide and iodide are more reactive and should be more suitable for some reactions. The laboratory synthesis of beryllium halides usually involves harsh conditions and complicated setups. A simple way to synthesize the compounds in the lab would, thus, be useful to expand the knowledge of beryllium chemistry.
Magnus R. Buchner, University of Marburg, Germany, and colleagues have developed a synthesis for anhydrous BeCl2, BeBr2, and BeI2 from the elements under mild conditions. The team condensed Cl2 at –77 °C into a Schlenk tube and connected this tube to a quartz ampoule containing Be powder. The valve was then opened to allow Cl2 gas to come into contact with Be and the metal was heated using a Bunsen burner to allow the formation of BeCl2. The product was purified via sublimation. The bromide was synthesized in an analogous manner.
BeI2 was synthesized by sealing Be powder and I2 in a quartz ampoule, heating the mixture at 200 °C to sublime the I2 and separate the reactants, and then heating the reaction vessel to 400 °C for 24 h. The reactants have to be separated because direct contact between Be and I2 at the high temperatures necessary for the reaction can cause explosions.
The researchers characterized the products using X-ray diffraction as well as infrared (IR) and Raman spectroscopy. The team also used density functional theory (DFT) calculations to calculate theoretical spectra for the beryllium halides. Using the results, the team was able to assign all observed bands in the IR and Raman spectra of the compounds for the first time.
- A facile synthesis for BeCl2, BeBr2 and BeI2,
Matthias Müller, Florian Pielnhofer, Magnus Richard Buchner,
Dalton Trans. 2018.
https://doi.org/10.1039/c8dt01756e