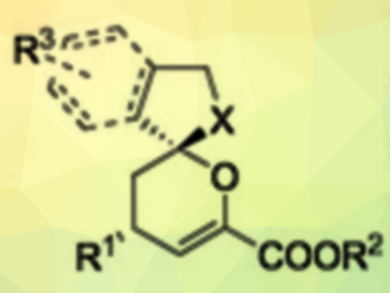
Chiral spiroketals are important structures in many natural products and bioactive compounds. However, it is still a challenge to synthesize such compounds in high yields with excellent diastereo- and enantioselectivities under mild conditions. Relay catalysis, which uses two different catalysts, allows reaction protocols that are not accessible through a single catalytic system. However, asymmetric versions of this approach are still very limited.
Qiang Kang, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, China, and colleagues have developed a bimetallic relay catalytic system combining an achiral Au(I) catalyst (AuCl·PPh3) and a chiral Rh(III) Lewis acid catalyst (pictured below). It catalyzes the reactions of alkynyl alcohols with β,γ-unsaturated α-ketoesters to give chiral spiroketals (pictured above) in excellent yields and with good diastereoselectivities and excellent enantioselectivities. The reactions are carried out in tetrahydrofuran (THF) at 35 °C under an argon atmosphere.
.jpg)
The researchers attribute the superior asymmetric induction compared with other Rh catalysts to the high steric bulk around the Rh center. The proposed reaction mechanism involves a gold‐catalyzed 5‐exo‐dig intramolecular hydroxylation cyclization of the alkynyl alcohol to give an enol ether intermediate. The β,γ‐unsaturated α‐ketoester is activated by the Rh catalyst. An inverse‐electron‐demand hetero‐Diels‐Alder reaction stereochemically controlled by the chiral Rh(III) complex then gives the spiroketal product. The catalyst system could also be used to synthesize chiral spiroaminals.
- Gold(I)/Chiral Rh(III) Lewis Acid Relay Catalysis Enables Asymmetric Synthesis of Spiroketals and Spiroaminals,
Jun Gong, Qian Wan, Qiang Kang,
Adv. Synth. Catal. 2018.
https://doi.org/10.1002/adsc.201800492