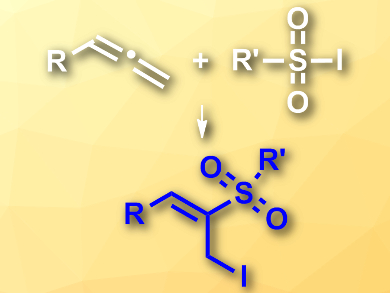
Allenes are reactive molecules that can be used in a variety of difunctionalization reactions to give useful synthetic intermediates. However, performing these reactions in a regioselective manner can be challenging.
Zhiguo Zhang, Guisheng Zhang, and colleagues, Henan Normal University, China, have developed a highly regioselective iodosulfonylation of allenes to give (E)-α-iodomethyl vinylsulfones (pictured). The team used sulfonyl iodides as combined iodine and sulfonyl sources, copper(I) iodide as a catalyst, and 1,10-phenanthroline as a ligand in dichloromethane (DCM) at room temperature.
The reaction gives the desired vinylsulfones in moderate to excellent yields and with high regio- and stereoselectivity and tolerates a range of functional groups. The researchers suggest that the reaction proceeds via a free-radical pathway. Both the allylic halide and the vinylsulfone group can be further transformed, which makes the products useful building blocks for organic synthesis.
- Copper-Catalyzed Difunctionalization of Allenes with Sulfonyl Iodides Leading to (E)-α-Iodomethyl Vinylsulfones,
Ning Lu, Zhiguo Zhang, Nana Ma, Conghui Wu, Guisheng Zhang, Qingfeng Liu, Tongxin Liu,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b01765