The electrochemical reduction of CO2 into useful chemical feedstocks is a promising and sustainable approach to store renewable energy. The major challenges of this reaction are the poor energy efficiency and product selectivity due to the large overpotential of CO2 activation, the complicated CO2 reduction pathway, and the competitive H2 evolution reaction (HER). Gold nanoparticles (AuNPs) with sizes below 5 nm are promising electrocatalysts to overcome these barriers.
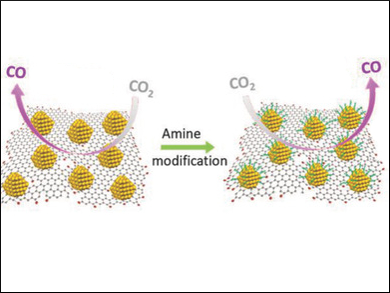
Caiyun Wang and Gordon G. Wallace, University of Wollongong, North Wollongong, Australia, and colleagues have used reduced graphene oxide (rGO) sheets as a platform for such ultrasmall AuNPs. The rGO-supported AuNPs were synthesized by adding a HAuCl4 solution to a rGO dispersion. The resulting composites have good catalytic activities and Faradaic efficiencies (32–60 %) for the CO2‐to‐CO conversion at moderate overpotentials of 450–600 mV.
The catalyst’s efficiency can be further improved to 59–75 % via a simple amine‐modification strategy. The team analyzed the effects of different amines, such as propylamine, oleylamine, and ethylenediamine (EDA) on the catalytic system. The researchers found that linear amines significantly improve the electrochemical reduction of CO2, whereas branched amines have the opposite effect and reduce CO formation. Oleylamine promoted CO selectivity most efficiently. The team attributes this effect to the strong interaction between oleylamine and gold and the optimal coverage of the AuNPs by the molecule.
- Engineering Surface Amine Modifiers of Ultrasmall Gold Nanoparticles Supported on Reduced Graphene Oxide for Improved Electrochemical CO2 Reduction,
Yong Zhao, Caiyun Wang, Yuqing Liu, Douglas R. MacFarlane, Gordon G. Wallace,
Adv. Energy Mater. 2018.
https://doi.org/10.1002/aenm.201801400