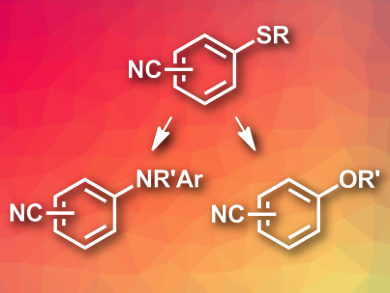
Diarylamines are commonly used, e.g, in dyes. There is a range of transition-metal-catalyzed syntheses for this type of compound, starting from aryl halides or boronic acids. Transition-metal-free reactions starting from other aryl derivatives, such as thioethers, would be useful to complement the existing synthetic pathways.
Wanxiang Zhao, Xue-Qiang Wang, Hunan University, Changsha, China, and colleagues have developed a transition-metal-free synthesis of diarylamines under mild conditions. The team used nitrile-substituted aryl alkyl thioethers (pictured) as starting materials and reacted them with aniline nucleophiles using KHDMS (potassium hexamethyldisilazane) as a base, and 1,4-dioxane as the solvent at 60 °C to give the desired diarylamines in good yields. The protocol can also be used to synthesize aryl alkyl ethers by replacing the anilines with alcohols and KHDMS with KOtBu (potassium tert-butoxide), as well as raising the reaction temperature to 80 °C.
The reactions have a broad substrate scope and tolerate a variety of functional groups. The researchers propose a nucleophilic aromatic substitution as the reaction mechanism. The team was able to perform the reaction on a gram scale. The nitrile group allows the products to be transformed further into useful chemical building blocks.
- Nucleophilic Amination and Etherification of Aryl Alkyl Thioethers,
Xia Wang, Yue Tang, Cheng-Yu Long, Wen-Ke Dong, Chenchen Li, Xinhua Xu, Wanxiang Zhao, Xue-Qiang Wang,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b01758