Hydrogen is an environmentally friendly energy carrier, capable of generating energy without the release of CO2 or other pollutants when used in fuel cells. Hydrogen is mostly produced using either methane steam reforming or water-gas reactions. New hydrogen generation pathways from renewable energy sources are of great interest to minimize the carbon footprint. A promising pathway is the photocatalytic splitting of water into hydrogen and oxygen using sunlight.
Reiner Sprick, University of Liverpool, Martijn Zwijnenburg, University College London, both UK, and colleagues have studied the photocatalytic activity of well-defined polymer photocatalysts and the effects of incorporating nitrogen or other heteroatoms. To this end, the researchers prepared a series of linear homo- and co-polymers that contain either nitrogen heterocycles or thiophene and compared them with heteroatom-free poly(p-phenylene).
The driving force for photocatalytic activity lies in both the material’s ability to oxidize the sacrificial electron donor and the overlap between the material’s absorption spectrum and the light source. Introducing electron-poor heterocyclic aromatics into unbranched conjugated polymers significantly increases the thermodynamic driving force for sacrificial electron donor oxidation, but decreases the light adsorption. In contrast, the introduction of electron-rich heterocycles increases the light absorption, but decreases the thermodynamic driving force for oxidation.
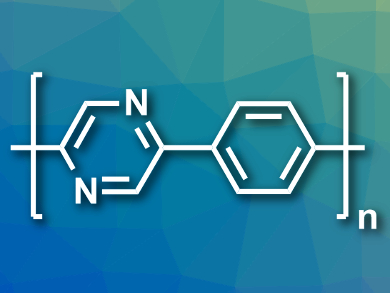
Overall, polymers based on pyridine and 2-phenylpyrazine (pictured), which absorb a reasonable amount of light and have a significant thermodynamic driving force for the oxidation of the sacrificial electron donor, were shown to provide the best hydrogen production of the polymers tested. According to the researchers, these results demonstrate that the optimization of only a single property is a poor strategy for improving the activity of photocatalysts.
- Nitrogen containing linear poly(phenylene) derivatives for photo-catalytic hydrogen evolution from water,
Reiner Sebastian Sprick, Liam Wilbraham, Yang Bai, Pierre Guiglion, Adriano Monti, Rob Clowes, Andrew I. Cooper, Martijn A. Zwijnenburg,
Chem. Mater. 2018.
https://doi.org/10.1021/acs.chemmater.8b02501