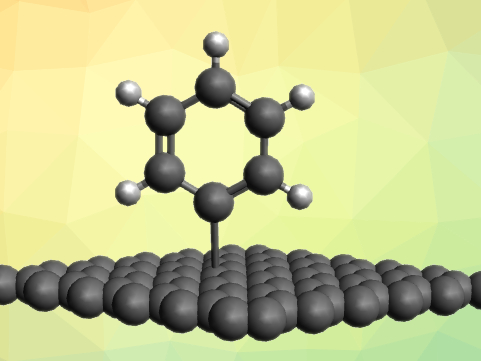
The functionalization of graphene is a useful tool for tailoring the material’s properties for different applications. However, graphene itself is not very reactive and thus, challenging to modify. Fluorinated graphene (FG) is a more promising precursor for many reactions. C–C coupling reactions involving graphene, in particular, could provide access to a wide variety of modified materials.
Xiangyang Liu and colleagues, Sichuan University, Chengdu, China, have developed the first Friedel–Crafts reaction of FG to give arylated graphene derivatives (pictured). The team prepared FG using a direct heat fluorination of graphene. The FG was then added to a solution containing an aryl reagent (e.g., methylbenzene, chlorobenzene, or polystyrene) and AlCl3. the reaction products could easily be isolated by filtration.
The reaction provides a high-density arylation of the graphene sheets in the products and proceeds under mild conditions. Most of the fluorine substituents are removed during the acylation reaction by a reductive defluorination. The developed method extends the substrate range of Friedel–Crafts reactions to 2D materials. According to the researchers, the prepared FG-polystyrene derivative, in particular, could be useful for the design of a graphene–polymer interface.
- The Friedel–Crafts reaction of fluorinated graphene for high-yield arylation of graphene,
Wenchuan Lai, Jiaxiang Liu, Longbo Luo, Xu Wang, Taijun He, Kun Fan, Xiangyang Liu,
Chem. Commun. 2018.
https://doi.org/10.1039/c8cc05762a