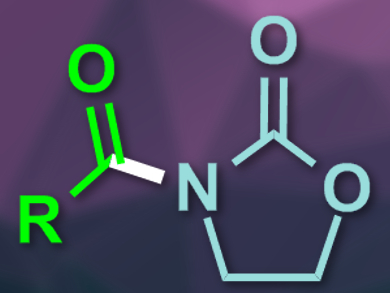
Functionalized oxazolidinones are useful chemical building blocks, e.g., for pharmaceuticals. N-acylated oxazolidinones are also widely used as chiral auxiliaries. Usually, highly reactive compounds are used for the synthesis of such compounds, e.g., strong bases and acid chlorides or anhydrides. The use of N-heterocyclic carbene (NHC) catalysis, together with an oxidant, could be a more environmentally friendly and milder alternative.
Henrik Sundén and colleagues, Chalmers University of Technology, Göteborg, Sweden, have developed the first N-acylation of synthetically useful oxazolidinones with aldehydes using oxidative NHC catalysis in air. The team used a range of aldehydes and oxazolidinones as substrates, different triazolium salts as NHC catalyst precursors, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) as base, iron(II)phthalocyanine and 3,3′,5,5′-tetra-tert-butyl-4,4′-diphenoquinone as electron transfer mediators (ETMs), and air as the oxidant at room temperature.
The reaction gives the desired N-acylated oxazolidinones in good to excellent yields and has a broad substrate scope. The team also used the approach for the synthesis of two natural products, piperlotine F and piperlotine G. According to the researchers, the method provides a sustainable way of using readily available aldehydes as acylation reagents.
- N-Acylation of Oxazolidinones via Aerobic Oxidative NHC Catalysis,
Linda Ta, Anton Axelsson, Henrik Sundén,
J. Org. Chem. 2018.
https://doi.org/10.1021/acs.joc.8b01723


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
