Information about chemical diffusion and diffusion coefficients is of importance for the design of ion-conducting layers in batteries, catalysts, and solid oxide fuel cells. CeO2 has a high oxygen diffusion coefficient, due to its fluorite structure. The wide variation of the reported values mirrors the experimental difficulties involved in the determination of the coefficient. Furthermore, the methods reported so far are mainly applicable for bulk material.
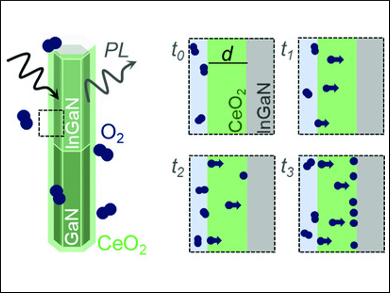
Paula Neuderth, University of Giessen, Germany, and colleagues have used ultrathin CeO2 layers with a thickness of 2–10 nm to determine the oxygen diffusion coefficient at relatively low temperatures between 50 °C and 200 °C. To this end, they used atomic layer deposition to coat InGaN/GaN nanowires in the desired ultrathin CeO2 layers (pictured). These nanowires show transient photoluminescence, which is quenched upon the adsorption of O2.
By observing the time-dependent decline of the photoluminescence at the InGaN/CeO2 interface, the team could calculate the oxygen diffusion coefficient for CeO2. The determined values are 1.5·10–16 cm2/s at 50 °C and 5.9·10–15 cm2/s at 200 °C. These coefficients are consistent with those measured at higher temperatures by experiments with isotope tracers or gas phase analysis.
- Optical Analysis of Oxygen Self-Diffusion in Ultrathin CeO2 Layers at Low Temperatures,
Paula Neuderth, Pascal Hille, Sara Martí-Sánchez, María de la Mata, Mariona Coll, Jordi Arbiol, Martin Eickhoff,
Adv. Energy Mater. 2018.
https://doi.org/10.1002/aenm.201802120