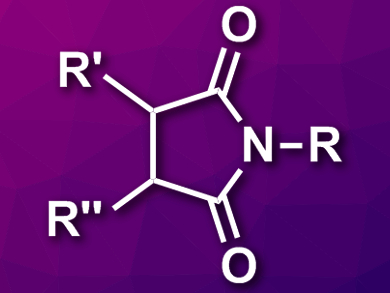
Substituted maleimides (pictured) are often found as substructures in bioactive compounds and useful materials. They are usually prepared by a reaction of amines with maleic anhydrides or related compounds. However, this usually leads to either nonsubstituted or symmetrically substituted products. Alternative pathways to polysubstituted maleimides would, thus, be useful.
Matthias Beller, Leibniz Institute for Catalysis, University of Rostock, Germany, and colleagues have developed a ligand-free palladium-catalyzed oxidative carbonylation reaction for the synthesis of a variety of substituted maleimides. The team used substituted alkynes and amines, as well as CO, as starting materials, PdCl2 as a catalyst, and air as a green oxidant. The reaction proceeds in toluene at 120 °C without the need for any potentially expensive ligands, additives, or co-oxidants.
The products are obtained in good to high yields of 63–95 % and the reaction has a broad substrate scope. The researchers propose a mechanism involving the generation of a palladium acyl complex from Pd(II), the amine, and CO, followed by the coordination of the alkyne’s triple bond to the metal. Then the alkyne and a second CO molecule are inserted Into the complex. Finally, the desired maleimide is eliminated and Pd(0) is re-oxidized to Pd(II) by air.
- Palladium-catalyzed aerobic oxidative carbonylation of alkynes with amines: a general access to substituted maleimides,
Ji Yang, Jiawang Liu, Ralf Jackstell, Matthias Beller,
Chem. Commun. 2018, 54, 10710–10713.
https://doi.org/10.1039/c8cc05802d
![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)


