Amines are functional groups that often require protecting groups during organic reactions. Carbamates such as t-butoxycarbonyl (Boc), benzyloxycarbonyl (Cbz), or 9-fluorenylmethoxycarbonyl (Fmoc) are commonly used amine protecting groups. Additional options for protecting groups with different deprotection conditions would be useful.
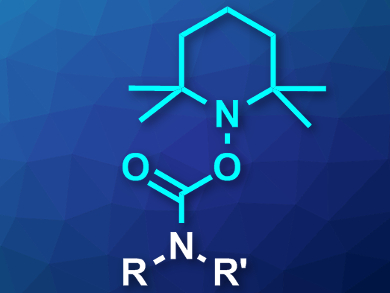
Peter Wipf, University of Pittsburgh, PA, USA, and colleagues have developed a new protecting group for primary, secondary, and heterocyclic amines: 2,2,6,6-tetramethylpiperidin-1-yloxycarbonyl (Tempoc, pictured). The group can easily be introduced using the acyl transfer reagent 4-nitrophenyl (2,2,6,6-tetramethylpiperidin-1-yl) carbonate (NPTC). This compound was synthesized by the reduction of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (Tempo) with sodium ascorbate and the O-acylation of the resulting hydroxylamine with p-nitrophenyl chloroformate.
The Tempoc-protected amines can be deprotected either under mild reductive conditions with Cu(I) compounds or by heating to 135 °C. This reactivity complements the commonly used protecting groups Boc (which is removed under acidic conditions) and Cbz (which is removed under hydrogenolytic conditions). According to the researchers, Tempoc could be very useful in organic synthesis.
- 2,2,6,6-Tetramethylpiperidin-1-yloxycarbonyl: A Protecting Group for Primary, Secondary, and Heterocyclic Amines,
Joseph R. Lizza, Maximilian Bremerich, Stephanie R. McCabe, Peter Wipf,
Org. Lett. 2018.
https://doi.org/10.1021/acs.orglett.8b02874