Efficient electrocatalytic water splitting requires good catalysts. Noble-metal oxides or platinum, for example, provide good efficiencies. However, rarity and high cost limit their use on a large scale. Alternatively, transition-metal dichalcogenides such as carbides and phosphides can be used. Nanostructured transition-metal phosphides show catalytic activity comparable to noble-metal catalysts.
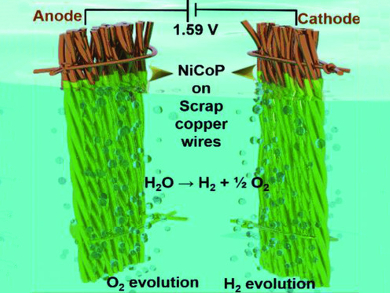
Sung Chul Yi, Hanyang University, Seoul, Republic of Korea, and colleagues have used copper from electronics waste, such as cables, as a catalytic substrate for overall water splitting. An effective bifunctional electrocatalyst was directly grown on the scrap copper wires by the electrodeposition of amorphous nickel cobalt phosphide films. The electrodeposition was performed in an electrolyte solution containing nickel sulfate, cobalt sulfate, sodium hypophosphite, sodium acetate, and boric acid.
The resulting catalyst (pictured) has a good electrochemical performance towards water splitting with a low overpotential of 1.59 V at 10 mA cm–2. The electrodes are long-term stable in an alkaline electrolyte. By the growth of bimetallic phosphide on the highly conductive copper substrate, fast electron transport and low contact resistances between the electrocatalyst and the current collector are achieved. The catalyst provides a productive way of reusing electronics waste.
- Harvesting Electronic Waste for the Development of Highly Efficient Eco-Design Electrodes for Electrocatalytic Water Splitting,
Vasanth Rajendiran Jothi, Ranjith Bose, Hashikaa Rajan, Chiyoung Jung, Sung Chul Yi,
Adv. Energy Mater. 2018.
https://doi.org/10.1002/aenm.201802615