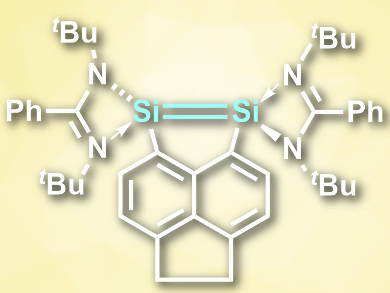
Compounds with double bonds between heavy group 14 elements such as silicon are rare and often need bulky substituents to stabilize them. Si═Si bonds, for example, are very reactive and, thus, generally unstable. In contrast to the lighter alkenes, disilenes (R2Si═SiR2) can easily dissociate into two singlet silylenes (R2Si:).
Arseni Kostenko and Matthias Driess, Technical University Berlin, Germany, have synthesized the hypercoordinated disilene with the longest Si═Si distance reported so far, 5,6-bis(amidinato silylenyl)acenaphtene (pictured). The compound was prepared by converting 5,6-dibromoacenaphthene to 5,6-dilithioacenaphthene and reacting this compound with (PhC(NtBu)2]SiCl. The Si═Si distance in the product is 2.623 Å, which was found using single-crystal X-ray diffraction.
According to the researchers, the Si=Si bond is composed of two donor–acceptor interactions between singlet silylenes, which are forced into close proximity by the rest of the molecule. The compound combines the reactivities of disilenes and of bis(silylene)s by taking part in [2+2] cycloadditions or forming bis(silylene) complexes with transition metals, respectively.
- Geometrically Compelled Disilene with λ4-Coordinate SiII Atoms,
Arseni Kostenko, Matthias Driess,
J. Am. Chem. Soc. 2018.
https://doi.org/10.1021/jacs.8b11393