Electrides are a type of ionic compound where localized electrons act as the anions. They are a class of materials with potential uses in catalysis and optoelectronics and can have zero-, one-, two-, or three-dimensional structures.
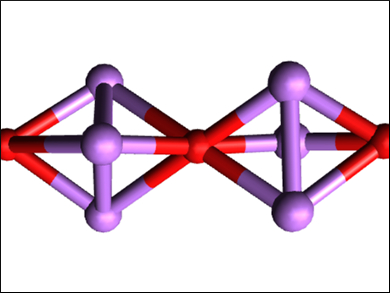
Hong Fang, Virginia Commonwealth University, Richmond, USA, and colleagues have used density functional theory (DFT) and density functional perturbation theory (DFPT) calculations, together with ab initio molecular dynamics (AIMD) simulations, to study whether a new class of hierarchical inorganic electrides could be made from Li3O super-alkali clusters. The team considered this possible because electrons can be easily detached from super-alkali clusters. This would create an electride if the detached electrons are localized in a repeating pattern. Under certain conditions, the clusters self-assemble into a one-dimensional, chain-like structure (pictured).
The calculations and simulations showed that the super-alkali clusters could be used as 1D electride building blocks, characterized by the presence of localized electrons—which possess a high charge density—along the chain. The researchers also showed that the stability of these electrides could be improved if they are embedded inside a boron nitride nanotube (BNNT) and that the resulting Li3O@BNNT structures could theoretically have effective reduction capabilities and anti-ferromagnetic properties.
- Super-alkalis as building blocks of one-dimensional hierarchical electrides,
Hong Fang, Jian Zhou, Puru Jena,
Nanoscale 2018.
https://doi.org/10.1039/c8nr07609j