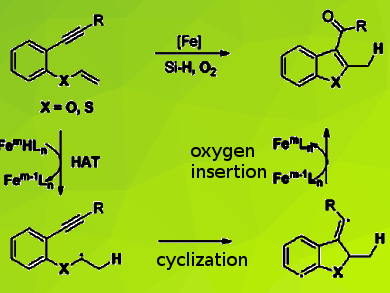
C3-Acylated benzofurans, benzothiophenes, and their related substituted heterocycles are structural motifs in many natural products and biologically active pharmaceutical molecules. Traditional methods to synthesize these compounds mainly focus on the cycloaddition reaction of phenol derivatives with 1,3-dicarbonyl compounds, the condensation of substituted acetophenones with dimethylformamide dimethyl acetal, and direct C3 acylation of benzofurans and benzothiophenes. These methods often suffer from the requirement of a stoichiometric amount of Lewis acids with limited substrate scope.
Xiao-Feng Xia and colleagues, Jiangnan University, Wuxi, People’s Republic of China, have used an iron-catalyzed hydrogen atom transfer (HAT) strategy, to develop an efficient reductive radical cyclization reaction of 1,6-enynes to give functionalized 3-acylbenzofurans and thiophenes. The reaction uses earth-abundant iron as the catalyst, silane as the hydrogen source, dioxygen as the oxygen source and ethanol as the solvent. The team confirmed a sequence involving hydrogen atom transfer (HAT)/cyclization and oxygen insertion by isotope experiments.
According to the researchers, their synthetic method consists of inexpensive and environmentally friendly reagents, the experimental procedure is simple and safe, and the strategy provides a viable synthetic approach to valuable carbonylated heteroaromatic compounds.
- Iron-catalyzed reductive cyclization reaction of 1,6-enynes for the synthesis of 3-acylbenzofurans and thiophenes,
Xiao-Feng Xia, Wei He, Guo-Wei Zhang, Dawei Wanga,
Org. Chem. Front. 2019.
https://doi.org/10.1039/C8QO01190G