1,n-Dicarbonyl groups occur in many natural products, biologically active compounds, and organic materials. A range of synthesis approaches is available for “shorter” derivatives such as 1,4- and 1,5-dicarbonyl compounds, while larger analogues such as 1,8-dicarbonyls are more difficult to make. Unsymmetric compounds are particularly challenging to prepare.
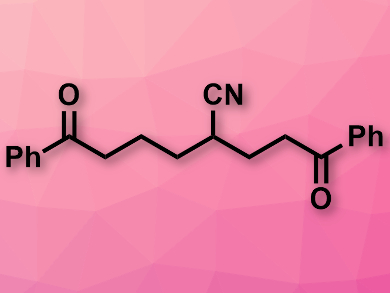
Chen Zhu and colleagues, Soochow University, Suzhou, China, have developed a method for the synthesis of unsymmetric 1,8.dicarbonyl compounds (example pictured), which is based on a coupling of cyclopropanols and bishomoallylic cyanohydrins under visible light. The team combined the substrates using Ir(ppy)2(dtbbpy)PF6 (ppy = 2-phenylpyridine, dtbbpy = 4,4′-di-tert-butyl-2,2′-dipyridyl) as a photocatalyst, BF3·Et2O as an additive, potassium persulfate as an oxidant, a mixture of dimethyl sulfoxide (DMSO) and water as the solvent, and a blue light-emitting diode (LED) as the light source.
The researchers obtained a range of multiply functionalized 1,8-diketones in moderate to good yields. According to the team, the reaction proceeds via oxidation of the cyclopropanol, ring opening, addition to the olefin group at the cyanohydrin component, and remote cyano migration.
- Visible-Light-Induced Consecutive C–C Bond Fragmentation and Formation for Synthesis of Elusive Unsymmetric 1,8-Dicarbonyl Compounds,
Meishan Ji, Zhen Wu, Chen Zhu,
Chem. Commun. 2019.
https://doi.org/10.1039/c9cc00378a


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
