There are few examples of mixed anionic compounds containing both nitride and hydride. One of these rare examples is Ca6Cr2N6H, in which Cr is trigonal-planar coordinated by nitrogen, while the hydride is surrounded by a distorted Ca octahedron and, thus, isolated from the transition metal. Compounds with a similar quarternary composition might also be stable.
Based on this theory, Tiglet Besara, National High Magnetic Field Laboratory, Tallahassee, FL, USA, Missouri State University, Springfield, USA, and colleagues have synthesized a new nitride-hydride, Ba3CrN3H. The team used a molten metal flux technique to prepare the compound. They combined freshly cut barium metal pieces, magnesium turnings, barium hydride powder, and chromium nitride powder in a stainless steel tube, which was welded shut and sealed in an evacuated quartz ampule. The ampule was heated to 850–1000 °C.
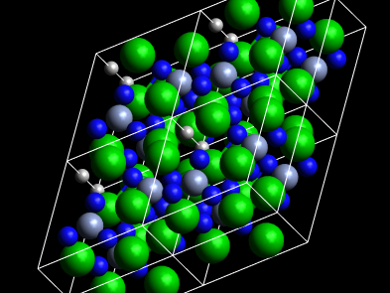
The researchers obtained the desired product as greenish hexagonal crystals, which decompose rapidly under ambient conditions. The compound was characterized using energy-dispersive spectroscopy (EDS), X-ray diffraction, and 1H solid-state nuclear magnetic resonance (NMR). The team found that the compound (pictured) contains trigonal planar [CrN3]5– units and octahedral [HBa6]11+ units. The presence of the hydride was confirmed by the NMR results.
- Ba3CrN3H: A New Nitride-Hydride with Trigonal Planar Cr4+,
Nathaniel W. Falb, Jennifer N. Neu, Tiglet Besara, Jeffrey B. Whalen, David J. Singh, Theo Siegrist,
Inorg. Chem. 2019.
https://doi.org/10.1021/acs.inorgchem.8b03367