Nitriles are useful intermediates in organic synthesis. They can easily be converted into a variety of other functional groups. Nitriles can be synthesized via a decarboxylative cyanation of carboxylic acids. However, existing approaches for this reaction require multiple steps or expensive catalysts.
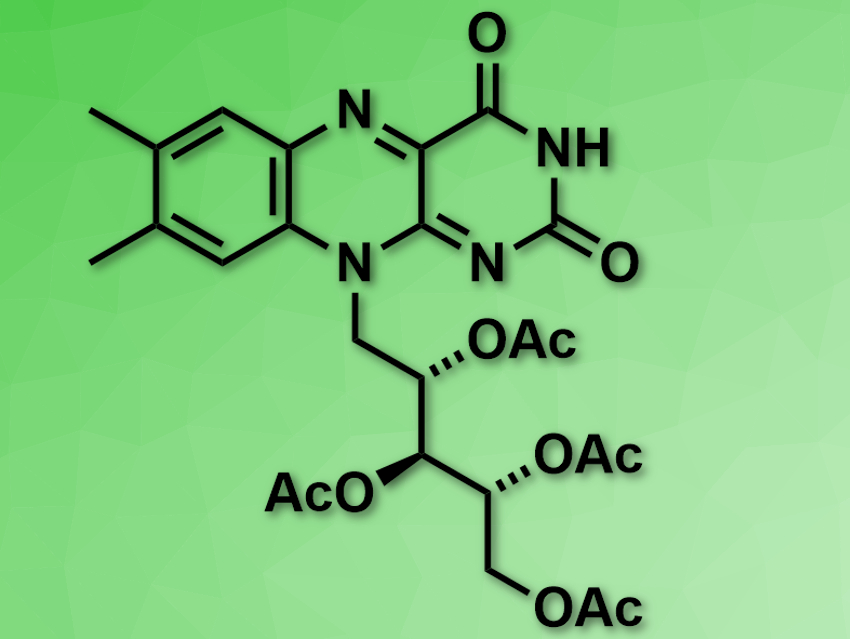
Jose C. Gonzalez-Gomez, Universidad de Alicante, Spain, and colleagues have developed a simple method for the decarboxylative cyanation of aliphatic carboxylic acids at room temperature. The method uses riboflavin tetraacetate as a low-cost, non-toxic photocatalyst, which can be prepared from riboflavin (vitamin B2) in a single step. Toluenesulfonyl cyanide (TsCN) was used as a cyanide source. The team was able to convert a range of carboxylic acids into the corresponding nitriles in moderate to good yields.
According to the researchers, the riboflavin tetraacetate promotes the oxidation of the carboxylic acid after activation with visible light, followed by a rapid decarboxylation of the generated acyloxyl radical, and trapping of the remaining alkyl radical with TSCN. The reaction tolerates a variety of functional groups.
- Decarboxylative Cyanation of Aliphatic Carboxylic Acids via Visible-Light Flavin Photocatalysis,
Nieves P. Ramirez, Burkhard König, Jose C. Gonzalez-Gomez,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b00064


![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)
