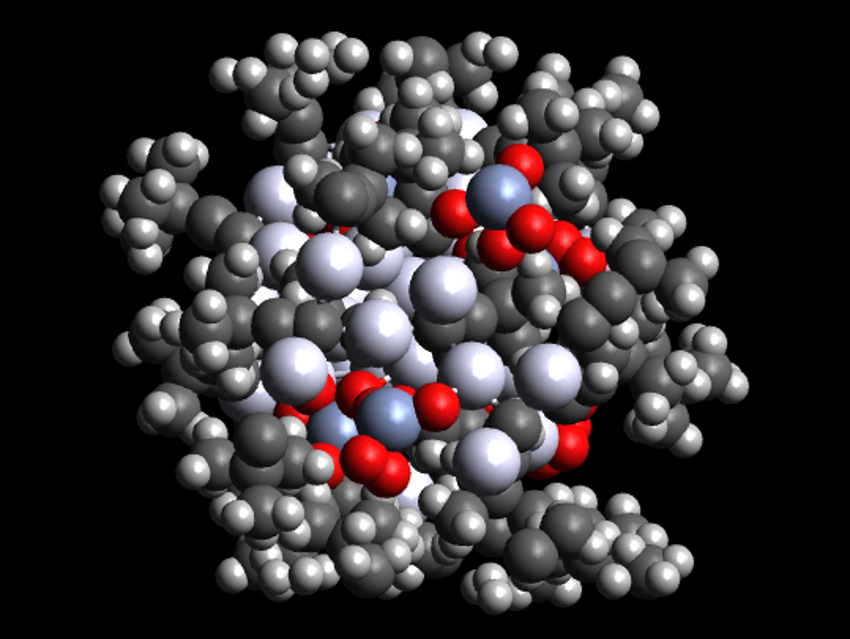
Metal nanoclusters have useful chemical and optical properties. They are usually protected by ligands which occupy the space between atoms and the bulk material. The capping ligands used are important for the clusters’ size and shape. Organic ligands such as thiolates or phosphines are often used. Inorganic ligands, usually halogens or chalcogens, are used less frequently.
Christine M. Aikens, Kansas State University, Manhattan, USA, Di Sun, Shandong University, Jinan, China, and colleagues have synthesized an atomically precise silver nanocluster, [Ag48(C≡CtBu)20(CrO4)7] (pictured), which is protected by both organic acetylide and inorganic chromate ligands. The team prepared the cluster by combining a mixture of tBuC≡CAg, K2Cr2O7, glutaric acid, and Ag2O with NaBH4 as a reductant in methanol at room temperature, followed by a solvothermal treatment at 70 °C.
The cluster consists of an inner cylinder-shaped Ag23 core, surrounded by an outer drum-shaped Ag25 shell. The chromate ions passivate the Ag23 cylinder and connect the core with the shell. The acetylide ligands cap the outer shell, each bridging three silver atoms. The researchers believe that the combination of organic ligands and inorganic oxo anions could open up new possibilities in the construction of silver nanoclusters.
- [Ag48(C≡CtBu)20(CrO4)7]: An Atomically Precise Silver Nanocluster Co-protected by Inorganic and Organic Ligands,
Shan-Shan Zhang, Fahri Alkan, Hai-Feng Su, Christine M. Aikens, Chen-Ho Tung, Di Sun,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b00703