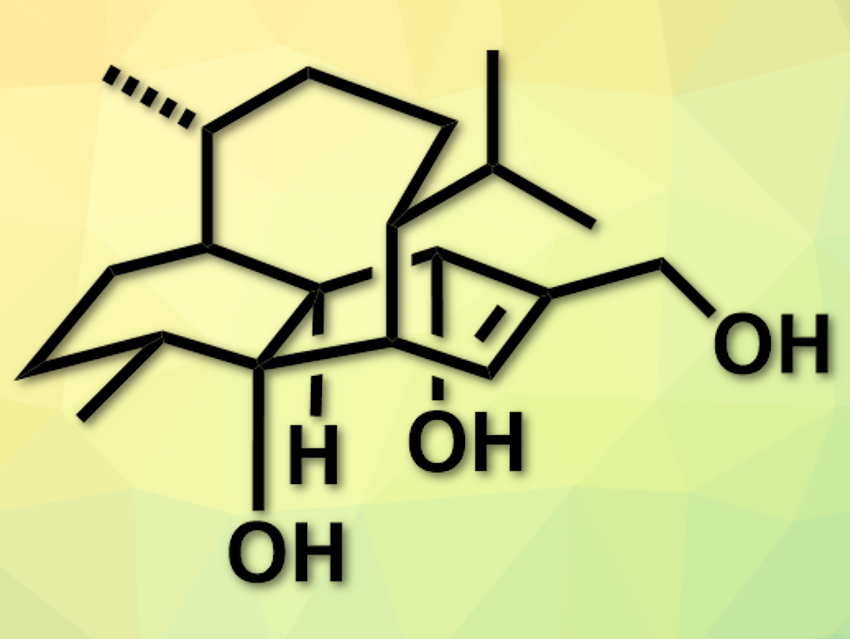
Vinigrol (pictured) is a natural product first isolated from a fungus. It has a tricyclic system with two six-rings and one eight-membered ring. It is pharmaceutically active, for example, against hypertension or platelet aggregation (a part of blood clotting). Due to the complex structure and many stereocenters, total syntheses of the compound usually require a large number of steps and are difficult to scale up.
Tuoping Luo, Peking University, Beijing, China, and colleagues have developed an efficient, scalable total synthesis of (−)-vinigrol. The team started from (S)-(−)-limonene on a 50 g scale and used only commercially available reagents in the course of the synthesis. Key steps are a hydroboration, a transannular Diels–Alder reaction, and several redox manipulations. The synthesis was optimized by minimizing the use of protecting groups.
The researchers obtained over 600 mg of (−)-vinigrol in 20 steps and with an overall yield of 1.4 %. This amount is sufficient for an investigation of the biological activities of (−)-vinigrol, which is ongoing according to the team.
- Scalable Total Synthesis of (−)-Vinigrol,
Xuerong Yu, Lianghong Xiao, Zechun Wang, Tuoping Luo,
J. Am. Chem. Soc. 2019, 141, 3440–3443.
https://doi.org/10.1021/jacs.9b00621