Chemiluminescence is the emission of light caused by a chemical reaction. If the reaction is induced by heating, the phenomenon is called thermochemiluminescence. Chemiluminescence had been known in solution but not in macroscopic crystalline solids.
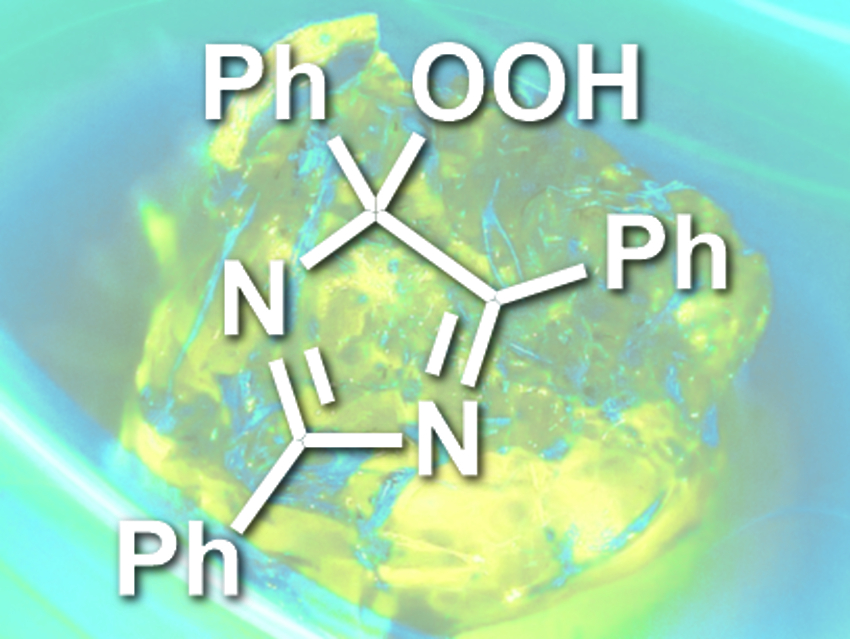
Panče Naumov, New York University Abu Dhabi, United Arab Emirates, and Harvard University, Cambridge, MA, USA, and colleagues have found that crystals can be thermochemiluminescent. The team heated crystals of lophine hydroperoxide (2,4,5-triphenyl-4-hydroperoxy-4H-isoimidazole, structure pictured) to about 115 °C and observed the emission of blue-green light.
The emission has a maximum at a wavelength of 530 nm and a low chemiluminescent quantum yield. The effect is caused by the decomposition of the peroxide. The progress of the reaction through the crystal could be monitored by observing the movement of the lit-up crystal region. The team also tested crystals of other organic peroxide classes, namely 1,2-dioxetanes, endoperoxides, and aroyl peroxides. They found that each of the tested compounds emits light upon heating to the respective decomposition temperature. The colors of the emitted light range from blue to green to red.
- Thermochemiluminescent peroxide crystals,
Stefan Schramm, Durga Prasad Karothu, Nathan M. Lui, Patrick Commins, Ejaz Ahmed, Luca Catalano, Liang Li, James Weston, Taro Moriwaki, Kyril M. Solntsev, Panče Naumov,
Nat. Commun. 2019.
https://doi.org/10.1038/s41467-019-08816-8