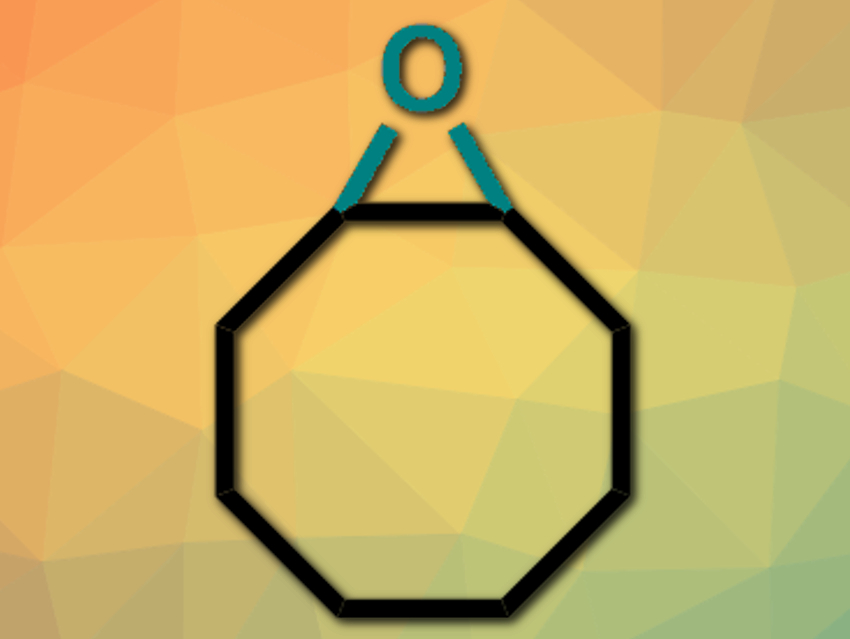
Epoxides are useful intermediates in synthetic organic chemistry. Existing methods for the epoxidation of allenes usually require harsh, hard-to-handle oxidants and/or produce stoichiometric amounts of byproducts.
Karthish Manthiram and colleagues, Massachusetts Institute of Technology (MIT), Cambridge, USA, have developed a sustainable, simple-to-operate electrocatalytic epoxidation protocol. The reaction uses manganese oxide nanoparticles as the catalyst and water as the source for oxygen atoms. Manganese oxide nanoparticles were drop-cast onto a hydrophilic carbon paper and then annealed at 400 °C. The resulting material was used as the working electrode, platinum foil was used as counterelectrode, and acetonitrile with added tetrabutylammonium tetrafluoroborate was used as the solvent for the electrochemical reaction of cis-cyclooctene and water. The reaction was performed at ambient conditions.
The desired cyclooctene oxide (pictured) was obtained with over 30 % Faradaic efficiency and with molecular hydrogen as the only stoichiometric byproduct. Control experiments with H218O confirmed that water acts as the oxygen source. According to the researchers, the developed approach could represent a new way to catalyze oxygen atom transfer reactions electrochemically.
- Epoxidation of Cyclooctene Using Water as the Oxygen Atom Source at Manganese Oxide Electrocatalysts,
Kyoungsuk Jin, Joseph H. Maalouf, Nikifar Lazouski, Nathan Corbin, Dengtao Yang, Karthish Manthiram,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b02345