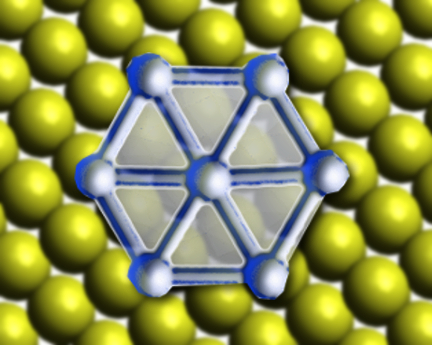
Borophene is an allotrope of boron, like graphene is an allotrope of carbon. Unlike most other 2D materials, borophene does not have a bulk counterpart, and cannot be created using exfoliation methods. Thus, understanding how the boron atoms grow into borophene is key to being able to synthesize it easily. Previous efforts to synthesize borophene have used a silver substrate.
Boris I. Yakobson, Rice University, Houston, TX, USA, Mark C. Hersam, Northwestern University, Evanston, IL, USA, Nathan P. Guisinger, Argonne National Laboratory, IL, USA, and colleagues have synthesized borophene on the (111) surface of a gold substrate using a thermal deposition method. The team investigated how the borophene forms using density functional theory (DFT) calculations and various characterization methods, including X-ray photoelectron spectroscopy (XPS), time-of-flight secondary ion mass spectrometry (TOF-SIMS), and scanning tunneling microscopy (STM).
When gold is used as the substrate, the boron atoms diffuse into the gold at high temperatures and form borophene “islands” as the systems cool. The process causes a rearrangement of the surface of the gold substrate. This rearrangement forms a trigonal network on the Au(111) surface, which promotes the growth of boron nanoclusters through nanotemplating. As the concentration of boron increases, the nanotemplating stops working and larger borophene islands are formed.
- Borophene Synthesis on Au(111),
Brian Kiraly, Xiaolong Liu, Luqing Wang, Zhuhua Zhang, Andrew J. Mannix, Brandon L. Fisher, Boris I. Yakobson, Mark C. Hersam, Nathan P. Guisinger,
ACS Nano 2019.
https://doi.org/10.1021/acsnano.8b09339