Enantioselective catalysis often requires transition-metal complexes. However, these metals can be expensive and toxic. Replacing them with main group elements could avoid these drawbacks. Like carbon, many of these elements can form stereogenic centers with a fixed configuration. Chirality at phosphorus or sulfur, for example, is well studied. Compounds with a configurationally stable stereogenic boron center, in contrast, are rare.
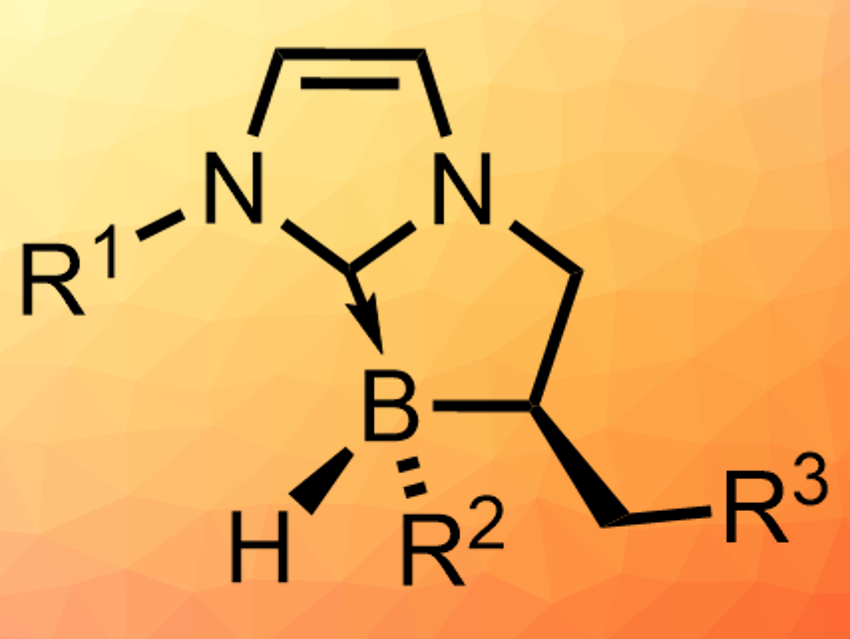
Olivier Chuzel and colleagues, Aix Marseille University, Marseille, France, have developed a diastereoselective synthesis of a range of stable chiral N-heterocyclic carbene (NHC)-boranes with stereogenic boron centers (pictured). The team started from cyclic NHC-boranes that are not stereogenic at boron, but have a defined carbon stereocenter next to the boron atom. These compounds were chlorinated at boron using hydrogen chloride. This intermediate is treated with a Grignard reagent to install R2 (pictured), which gives the desired products in high yields (up to 93 %) and, in many cases, as a single diastereomer.
The researchers propose that the last reaction proceeds via an SRN1 mechanism, which has a radical intermediate that is preferentially attacked from one side. This explains the diastereoselectivity. The chiral NHC-boranes could be useful, e.g., for metal-free enantioselective reduction catalysis.
- Highly diastereoselective preparation of chiral NHC-boranes stereogenic at boron atom.,
Olivier Chuzel, Clara Aupic, Amel Abdou-Mohamed, Calrlotta Figliola, Paola Nava, Beatrice Tuccio, Gaëlle Chouraqui, Jean-Luc Parrain,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc01454c