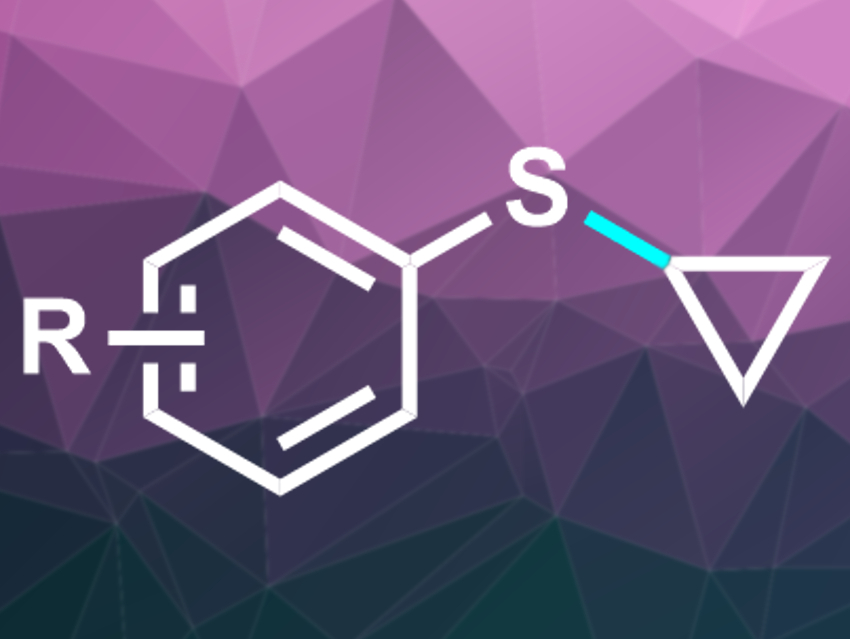
Aryl cyclopropyl sulfides (pictured) and their derivatives are found in many biologically active molecules and can be useful intermediates in synthetic organic chemistry. These compounds can be prepared. e.g., from thiophenols and cyclopropyl bromide or from aryl fluorides and cyclopropanethiol. However, these reactions usually require harsh conditions and in some cases, need electron-withdrawing substituents on the aryl ring.
Alexandre Gagnon and colleagues, Université du Québec à Montréal (UQAM), Canada, have developed a copper-catalyzed protocol for the synthesis of aryl cyclopropyl sulfides. The team reacted thiophenols with cyclopropylboronic acid at 70 °C, using Cu(OAc)2 as a catalyst, bipyridine as a ligand, cesium carbonate as a base, and 1,2-dichloroethane (DCE) as a solvent.
The reaction gave moderate to excellent yields of the desired aryl cyclopropyl sulfides within 16 hours. The reaction tolerates both electron-withdrawing and electron-donating groups at the aryl reactant. Substituents in ortho-position, however, lead to lowered yields.
- Synthesis of aryl cyclopropyl sulfides through copper-promoted S-cyclopropylation of thiophenols using cyclopropylboronic acid,
Emeline Benoit, Ahmed Fnaiche, Alexandre Gagnon,
Beilstein J. Org. Chem. 2019, 15, 1162–1171.
https://doi.org/10.3762/bjoc.15.113