There is a large amount of research on hydrogen evolution catalysts. This is due to the hope that they could enable a sustainable, hydrogen-based fuel economy. However, molecular hydrogen evolution catalysts are not yet useable on a large, industrial scale. These catalysts are designed to perform the reduction of protons to dihydrogen. This might make it possible to use them for other, smaller-scale reactions: reductions in organic chemistry.
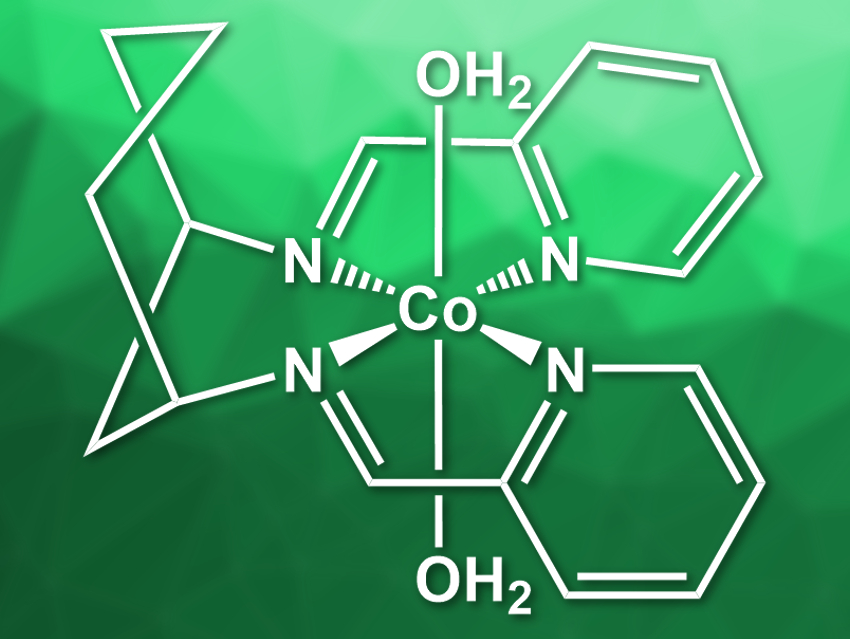
Cobalt bis-iminopyridines, for example, are low-cost molecular hydrogen evolution catalysts. John Fielden, University of East Anglia, UK, and colleagues have used cobalt bis-iminopyridine derivatives for the reduction of acetonitrile to ethylamine. The team synthesized two Co complexes: [Co(DDP)(H2O)2](NO3)2 (pictured, DDP = cis-[1,3-bis(2-pyridinylenamine)]cyclohexane)) and [Co(cis-DDOP)(NO3)](NO3) (cis-DDOP = cis-3,5-bis[(2-pyridinyleneamin]-trans-hydroxycyclohexane)). The complexes were prepared by mixing the ligands with cobalt(II) nitrate in methanol.
The team found that [Co(DDP)(H2O)2](NO3)2 can electrocatalyze the reduction of acetonitrile to ethylamine. This reaction is favored over the production of dihydrogen. [Co(cis-DDOP)(NO3)](NO3), in contrast, favors H2 production. The researchers attribute this effect to the hydroxy group in the second catalyst, which coordinates to cobalt and makes it more difficult to coordinate acetonitrile during the reaction. According to the team, this work shows that catalysts designed for hydrogen evolution could have alternative uses in organic chemistry.
- Cobalt-based molecular electrocatalysis of nitrile reduction: evolving sustainability beyond hydrogen,
Simon N. Child, Radoslav Raychev, Nathan Moss, Benjamin Howchen, Peter N. Horton, Christopher C. Prior, Vasily S. Oganesyan, John Fielden,
Dalton Trans. 2019.
https://doi.org/10.1039/c9dt00773c