Benzene is known for its stability. While its C–H bonds in arenes can be activated using several different methods, breaking their C–C bonds is particularly difficult. Usually, reactive species such as carbenes that can only be generated in situ are required for this type of reaction.
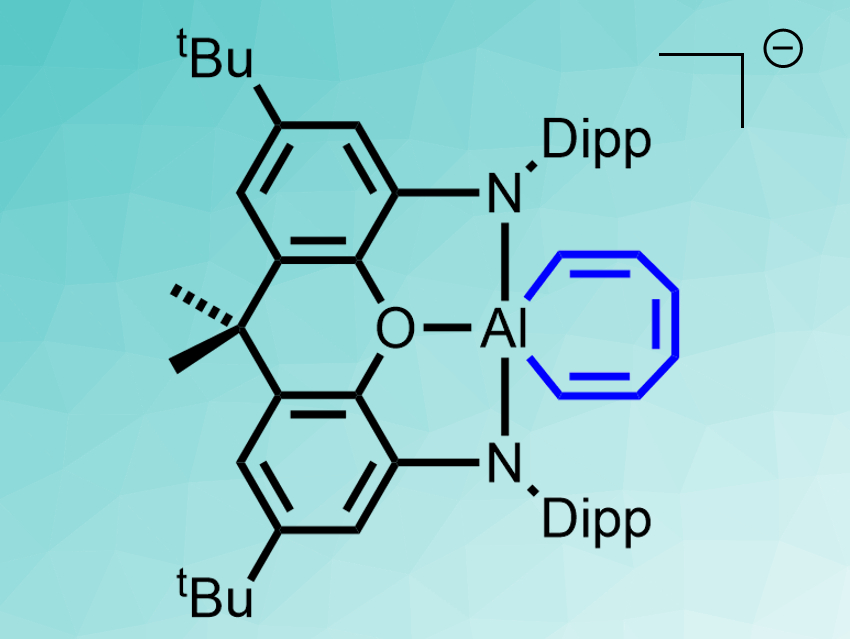
Jose M. Goicoechea, Simon Aldridge, University of Oxford, UK, and colleagues have found an isolable metal complex that can reversibly activate a C–C bond in benzene at room temperature. The team used the complex salt [K(2.2.2-crypt)][(NON)Al] (NON = 4,5-bis(2,6-diisopropyl-anilido)-2,7-di-tert-butyl-9,9-dimethylxanthene), which is stable enough to be isolated and studied using X-ray crystallography. The anionic aluminium complex of this salt reacts with benzene at room temperature in the dark, giving a seven-membered AlC6H6 metallacycle (pictured) in a yield of 95 %.
The researchers showed that this C–C bond breaking is reversible: Heating the metallacycle to 80 °C leads to the formation of benzene. The team also studied the reactivity of the metallacycle and found that it can be used to synthesize acyclic derivatives such as trienes from the original benzene.
- Reversible, Room-Temperature C–C Bond Activation of Benzene by an Isolable Metal Complex,
Jamie Hicks, Petra Vasko, Jose M. Goicoechea, Simon Aldridge,
J. Am. Chem. Soc. 2019.
https://doi.org/10.1021/jacs.9b05925