The hydrogenation of nitriles with H2 is an atom-economical path to amines that is used in industrial processes. Heterogeneous catalysts are particularly useful in industry because they are easy to separate from the reaction mixture and can be reused. Commonly, metals such as Pd, Co, Ni, or Fe are used in these processes. However, these catalysts can suffer from low selectivity and sometimes need harsh reaction conditions. Complexes of metals such as Ru with well-defined ligands, which are usually used in homogeneous catalysis, usually are more selective and work under milder conditions.
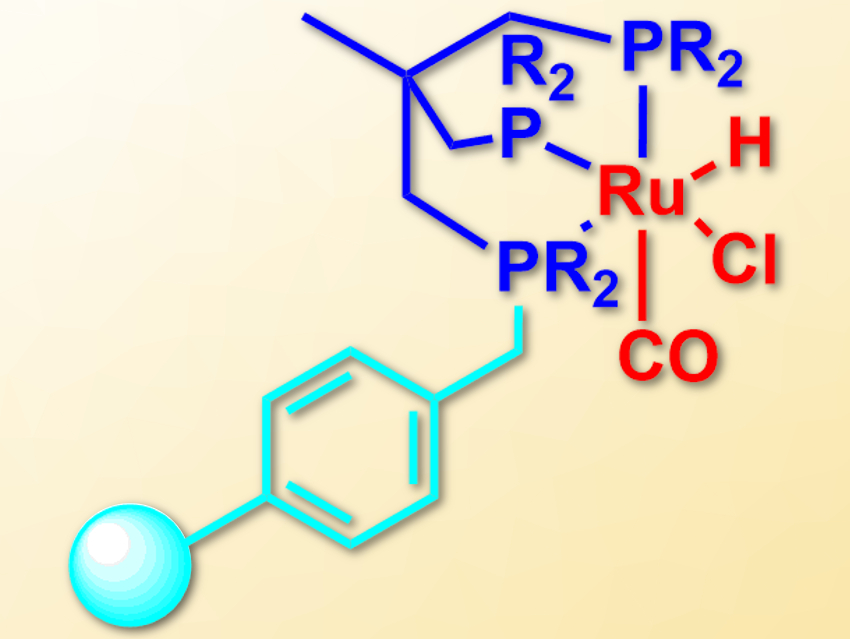
Paul C. J. Kamer, Leibniz Institute for Catalysis, University of Rostock, Germany, and colleagues have immobilized triphos-type ligands on a cross-linked polystyrene resin. For this, the team first bound a secondary phosphine to the resin, which was converted to a lithium phosphide using lithium diisopropylamide (LDA). This intermediate was reacted with 1,1,1-tris(chloromethyl) ethane to give a phosphine dimethylenechloride intermediate. This compound was converted to the desired triphosphine ligand using an excess of another secondary lithium phosphide.
The resulting immobilized ligands form ruthenium complexes (pictured) when treated with [RuHCl(PPh3)3CO]. The supported complexes can be used as heterogeneous catalysts for the reduction of nitriles to amines with H2 under mild conditions. The catalyst can also be used in flow chemistry, which is the first use of a Triphos-type catalyst in a continuous flow process. It provides a high catalyst lifetime of more than 195 hours.
- Facile Synthesis of Supported Ru-Triphos Catalysts for Continuous Flow Application in Selective Nitrile Reduction,
Paul Kamer, Robert Konrath, Frank Heutz, Norbert Steinfeldt, Nils Rockstroh,
Chem. Sci. 2019.
https://doi.org/10.1039/c9sc01415b