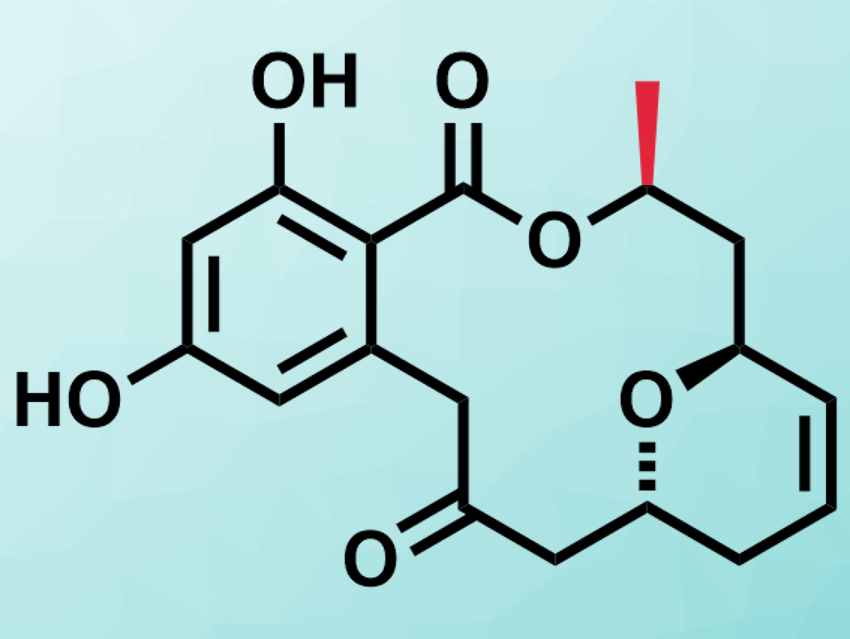
Resorcylic acid lactones (RALs) are a class of natural products which consist of a resorcin moiety and a 12- or 14-membered macrolactone. Monocillin VII is one example of a RAL and was isolated from fungi in 2017 [1]. It has antifungal and cytotoxic properties.
Debendra K. Mohapatra, CSIR – Indian Institute of Chemical Technology, Hyderabad, and colleagues have performed the first asymmetric total synthesis of monocillin VII. Based on the results, the team revised the previously proposed configuration of the compound. The team started from a known chiral pure epoxide to ensure the correct configuration. They connected the resorcin precursor and the macrolactone precursor via a Sonogashira coupling. The formation of the macrolactone involved the conversion of an alkyne in the structure to an alkyne–dicobalt carbonyl complex. This distorted the angle of the alkyne from 180° to ca. 140° and allowed the efficient formation of the lactone.
The analytic results for the synthesized monocillin VII deviated from those reported for the natural product. This led the researchers to suspect that the absolute configuration of the compound had been wrongly assigned. They prepared the epimer with the methyl group at the 10′ position in the (S)-configuration (pictured in red), opposite of the reported structure. For this isomer, the team found a good agreement with the spectral data reported for the natural product. Thus, the structure of monocillin VII had to be corrected.
- Total Synthesis and Structural Revision of Monocillin VII,
N. Arjunreddy Mallampudi, Beduru Srinivas, Jithender G. Reddy, Debendra K. Mohapatra,
Org. Lett. 2019.
https://doi.org/10.1021/acs.orglett.9b02075
Reference
- [1] Antifungal and Cytotoxic β-Resorcylic Acid Lactones from a Paecilomyces Species,
Liangxiong Xu, Ping Wu, Jinghua Xue, Istvan Molnar, Xiaoyi Wei,
J. Nat. Prod. 2017, 80, 2215–2223.
https://doi.org/10.1021/acs.jnatprod.7b00066