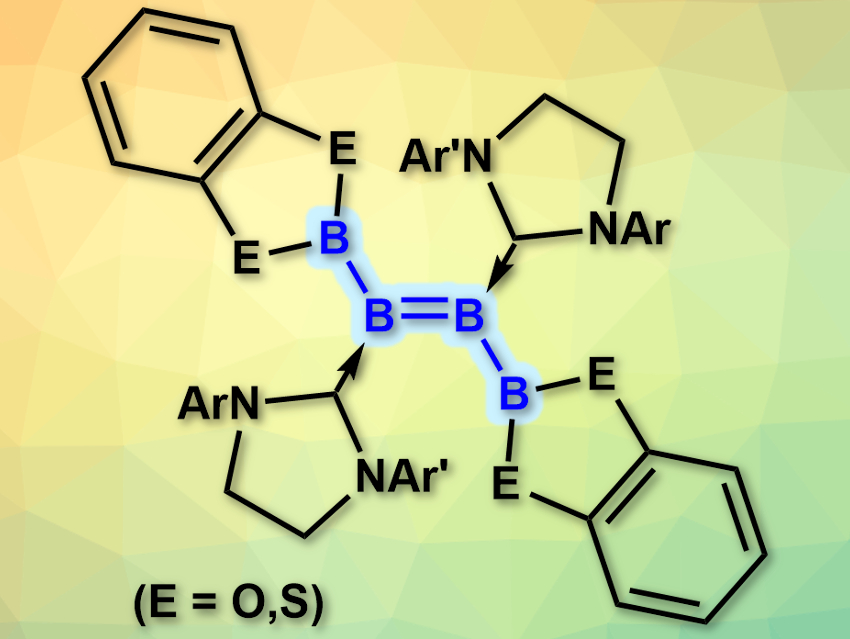
Boron compounds tend to form clusters due to the electron deficiency of the element. This makes it difficult to synthesize defined, small boron compounds such as the analogues of small organic molecules. There are some syntheses for these compounds (e.g., linear tri- and tetraboranes), however, they require harsh conditions and do not tolerate many functional groups.
Holger Braunschweig, University of Würzburg, Germany, and colleagues have developed a synthesis of diboryldiborenes (pictured, boron analogues of 2-butene) under mild conditions. The team reacted doubly NHC-stabilized diborynes (NHC = N-heterocyclic carbene) with either bis(catecholato)diboron or bis(dithiocatecholato)diboron without a catalyst at room temperature in benzene. The B–B triple bond in the diboryne was diborated to give the desired products.
Depending on the substituents at the NHC, some of the products are stable for days in the solid state in air and even suspended in water. This could allow their use in, e.g., molecular electronic materials. According to the researchers, the reaction provides a new path for the challenging construction of electron-precise B–B bonds.
- Mild synthesis of diboryldiborenes by diboration of B–B triple bonds,
Tobias Brückner, Rian D. Dewhurst, Theresa Dellermann, Marcel Müller, Holger Braunschweig,
Chem. Sci. 2019, 10, 7375–7378.
https://doi.org/10.1039/c9sc02544h